CHEM 121L ACID/BASE II
CHEM 121L ACID/BASE II
CHEM 121L ACID/BASE II
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
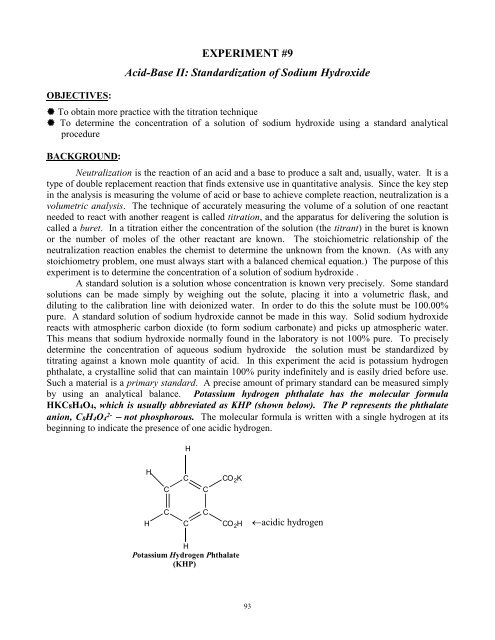
OBJECTIVES:EXPERIMENT #9Acid-Base <strong>II</strong>: Standardization of Sodium Hydroxide To obtain more practice with the titration technique To determine the concentration of a solution of sodium hydroxide using a standard analyticalprocedureBACKGROUND:Neutralization is the reaction of an acid and a base to produce a salt and, usually, water. It is atype of double replacement reaction that finds extensive use in quantitative analysis. Since the key stepin the analysis is measuring the volume of acid or base to achieve complete reaction, neutralization is avolumetric analysis. The technique of accurately measuring the volume of a solution of one reactantneeded to react with another reagent is called titration, and the apparatus for delivering the solution iscalled a buret. In a titration either the concentration of the solution (the titrant) in the buret is knownor the number of moles of the other reactant are known. The stoichiometric relationship of theneutralization reaction enables the chemist to determine the unknown from the known. (As with anystoichiometry problem, one must always start with a balanced chemical equation.) The purpose of thisexperiment is to determine the concentration of a solution of sodium hydroxide .A standard solution is a solution whose concentration is known very precisely. Some standardsolutions can be made simply by weighing out the solute, placing it into a volumetric flask, anddiluting to the calibration line with deionized water. In order to do this the solute must be 100.00%pure. A standard solution of sodium hydroxide cannot be made in this way. Solid sodium hydroxidereacts with atmospheric carbon dioxide (to form sodium carbonate) and picks up atmospheric water.This means that sodium hydroxide normally found in the laboratory is not 100% pure. To preciselydetermine the concentration of aqueous sodium hydroxide the solution must be standardized bytitrating against a known mole quantity of acid. In this experiment the acid is potassium hydrogenphthalate, a crystalline solid that can maintain 100% purity indefinitely and is easily dried before use.Such a material is a primary standard. A precise amount of primary standard can be measured simplyby using an analytical balance. Potassium hydrogen phthalate has the molecular formulaHKC8H4O4, which is usually abbreviated as KHP (shown below). The P represents the phthalateanion, C8H4O4 2- not phosphorous. The molecular formula is written with a single hydrogen at itsbeginning to indicate the presence of one acidic hydrogen.HHCCCCO 2 KHCCCCO 2 Hacidic hydrogenHPotassium Hydrogen Phthalate(KHP)93
EXPERIMENT #9STANDARDIZATION OF NaOHTo perform the standardization, a solution of sodium hydroxide is placed in a buret, and aknown mass of KHP is placed in an Erlenmeyer flask and dissolved in water. The basic solution isadded to the flask until the KHP has been consumed. This point -- the end point -- can be determinedby the change in color of an indicator placed in the flask at the start of the titration. Indicators aresubstances that undergo specific color changes under certain conditions in solution. An indicator thatworks very well in this experiment is phenolphthalein. Phenolphthalein the active ingredient inEXLAX is colorless until the KHP is consumed. The slightest excess of sodium hydroxide turnsthe indicator pink. The end point is observed as the first faintest pink color that persists for 30 seconds.At the end point the known quantity of KHP is stoichiometrically equivalent to the sodium hydroxidedelivered by the buret.The balanced equation for the reaction of sodium hydroxide with KHP isKHP(aq) + NaOH(aq) NaKP(aq) + HOH(l) (1)Since one mole of KHP has one mole of acidic protons and one mole of NaOH has one mole ofhydroxide ions, the coefficients of each reactant in equation (1) are the same. In other words one moleof KHP is stoichiometrically equivalent to one mole of NaOH. Therefore, at the endpoint of thetitration,moles KHP = moles NaOH (2)The KHP was weighed out, somass KHPmole KHP (3)molar mass KHPSince the sodium hydroxide was measured volumetrically,Substituting (3) and (4) into (2), we getmole NaOH = MolarityNaOH x VolumeNaOH (4)mass KHP= MolarityNaOH x VolumeNaOH (5)molar mass KHPRearranging to solve for the concentration of sodium hydroxide, we getmass KHPxmolar mass KHP1VolumeNaOH Molarity(6)NaOHThus, the molarity of the sodium hydroxide solution is equal to the mass of KHP divided by theproduct of the molar mass of KHP and the volume of sodium hydroxide solution used to reach the endpoint. Remember that volume has units of liters and molarity has units of moles per liter.Perform at least three titrations to standardize the sodium hydroxide solution, but do as many asit takes to get a relative standard deviation (rsd) of less than 5 ppt. [Check with your instructor.]94
EXPERIMENT #9STANDARDIZATION OF NaOHExample: What is the molarity of a sodium hydroxide solution if 35.745 mL is required to titrate asolution of 1.070 g of KHP?moles NaOH = moles KHPmoleKHPmass KHPmolar mass KHP1.070gmoleKHP204.22gmolmoleKHP 0.005239 molmol NaOHmolarity NaOH Liters NaOH0.005239 molmolarity NaOH 0.03575Lmolarity NaOH 0.1466 MEXPERIMENTAL PROCEDURE: (Work individually.)Sodium hydroxide is classified as a corrosive and can easily do irreversible damage to youreyes. Please make sure you wear your safety goggles throughout the experiment.1. The sodium hydroxide solution has been prepared and is stored in a large plastic container. Obtaina clean dry beaker and take no more that 75 mL of solution. If this is insufficient for your needs,you can always get more later. NEVER RETURN REAGENTS TO THE ORIGINALCONTAINER.2. Obtain a buret and run deionized water through the buret to check that the stopcock does not leak.Also note if the water runs freely down the sides of the buret without leaving droplets. If dropletsare left behind or if the curved meniscus is not symmetrical, the buret needs to be cleaned withsoap solution, a buret brush , and lots of "elbow grease." Thoroughly rinse the buret withdeionized water.95
EXPERIMENT #9STANDARDIZATION OF NaOH3. A buret must be thoroughly rinsed with the titrant it is to deliver. When the buret has drainedcompletely after step 2, close the stopcock and add 2-3 mL of the sodium hydroxide solution.Hold the buret almost horizontally, while keeping the open end over the sink or a large beaker (incase some titrant spills). Rotate the buret in order to rinse the inside walls with titrant. Hold theburet vertically with the stopcock down, open the stopcock to rinse the tip, and let the titrant draininto the beaker. Repeat this rinsing process two more times. The buret may now be filled withtitrant. The used titrant in the beaker can be neutralized with dilute acid and poured down the sinkwith copious amounts of water.4. Hold the buret vertically over the sink, open the stopcock, and with a flick of the wrist (watch yourinstructor) remove any air bubbles trapped near or below the stopcock. It is important to removeair bubbles for precise work. Replenish the buret with more titrant so that the level of liquid isclose, but below, the top graduation mark of the buret.5. A buret is read to two decimal places. The second decimal place is a "best guess" on the part ofthe operator. As you gain more experience, you will find it easier to "guess" that second decimalplace. As a guide read the second decimal place as .x0, .x2, .x5, or .x8. Place a white backgroundbehind the buret, find the bottom of the meniscus, and read the graduations at eye level.6. Weigh out to the nearest milligram or better (How many decimal places?) three samples of primarystandard KHP each between each between 0.4 and 0.6 g, place each sample into a 250 mlErlenmeyer flask, and carefully label each flask. The mass of each sample should be different,but each sample should weigh between 0.4 and 0.6 g. (If the lines to the balances are long, weighout one sample and perform the titration before weighing out the second sample.) Dissolve eachsample in approximately 100 mL of deionized water. Swirl the flasks and warm gently with yourhands or a warm water bath to completely dissolve the KHP. Add two to three drops ofphenolphthalein to each flask before starting the titration.7. Slowly add the titrant to one flask, while gently swirling its contents. As the sodium hydroxidesolution is added, a pink color appears where the drops of the base come in contact with thesolution. The color disappears with swirling, but near the end point the color disappears moreslowly. Near the end point the titrant must be added drop by drop or even one half drop by onehalf drop. It is most important that the flask be swirled constantly throughout the entire titration toensure homogeneity. The faintest pink color that persists in front of a white background for 30 -60 seconds after swirling indicates the end point has been reached. Reread the buret after this timeframe and record on your data sheet. Any drop adhering to the buret tip should be added to theflask. Pay close attention as your instructor demonstrates the technique. Repeat the procedurewith your other samples.96
NAME________________________________ Section_______ Date__________DATA AND OBSERVATIONS: Standardization of NaOHDeterminationsFirst Second Third__________________________________________ _____________ _______________________________________________________ _____________ _____________Mass of KHP, g_____________ _____________ _____________Final volume of NaOH, mL_____________ _____________ _____________Initial volume of NaOH, mL_____________ _____________ _____________Volume of NaOH used, mL_____________ _____________ _____________Moles KHP_____________ _____________ _____________Moles NaOH_____________ _____________ _____________Molarity of NaOH_____________ _____________ _____________Average molarity_____________Calculations97
NAME________________________________ Section_______ Date__________ADDITIONAL ASSIGNMENT I: Standardization of NaOH1. If 50.0 mL of NaOH solution are required to react completely with 0.210 g KHP, what is themolarity of the NaOH solution?2. A 3.664 g sample of a monoprotic acid was dissolved in water and required 20.27 mL of a 0.1578M NaOH solution for neutralization. Calculate the molar mass of the acid.3. What is the molarity of a NaOH solution if 15.25 mL of it is required to neutralize 0.7735 g ofKHP?99
4. A 0.7026 g sample of an unknown solid acid requires 40.96 mL of 0.1158 M NaOH forneutralization to a phenolphthalein end point.a. How many moles of NaOH are used?b. How many moles of H + are there in the solid acid?c. How many grams of acid are present per mole of H + ? (This number is the equivalent massof the acid.)100
NAME________________________________ Section_______ Date__________ADDITIONAL ASSIGNMENT <strong>II</strong>: Standardization of NaOH1. In the titration of an impure sample of KHP, it was found that 29.4 mL of 0.100 M NaOH wasrequired to react completely with 0.745 g of sample. What is the percentage of KHP in thissample?2. What is the percentage of KHP in a sample if 2.537 g requires 32.77 mL of 0.1466 M NaOH toneutralize it?101
3. Calculate the volume in milliliters of a 1.420 M NaOH solution required to titrate the followingsolutions:(a) 25.00 mL of 2.430 M HCl solution(b) 25.00 mL of a 4.500 M H2SO4 solution(c) 25.00 mL of a 1.500 M H3PO4 solution102