Chapter 15 – Chemical Equilibrium
Chapter 15 – Chemical Equilibrium
Chapter 15 – Chemical Equilibrium
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
3<br />
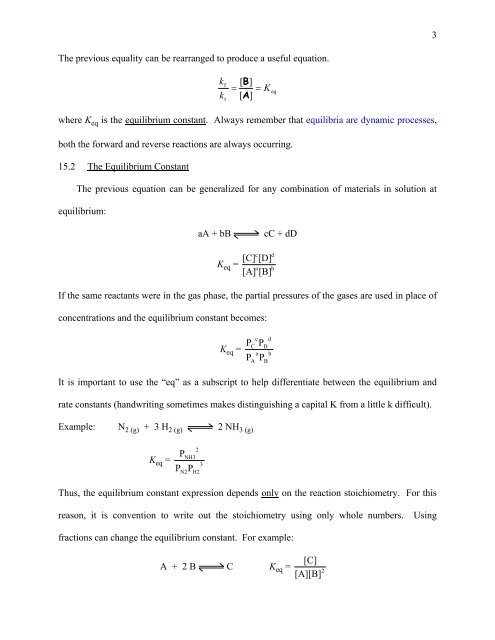
The previous equality can be rearranged to produce a useful equation.<br />
k<br />
k<br />
f<br />
r<br />
[ B]<br />
= = K<br />
[ A ]<br />
eq<br />
where K eq is the equilibrium constant. Always remember that equilibria are dynamic processes,<br />
both the forward and reverse reactions are always occurring.<br />
<strong>15</strong>.2 The <strong>Equilibrium</strong> Constant<br />
The previous equation can be generalized for any combination of materials in solution at<br />
equilibrium:<br />
aA + bB<br />
K eq =<br />
c<br />
[C] [D]<br />
a<br />
[A] [B]<br />
cC + dD<br />
d<br />
b<br />
If the same reactants were in the gas phase, the partial pressures of the gases are used in place of<br />
concentrations and the equilibrium constant becomes:<br />
K eq =<br />
P<br />
P<br />
c d<br />
C<br />
PD<br />
a b<br />
A<br />
PB<br />
It is important to use the “eq” as a subscript to help differentiate between the equilibrium and<br />
rate constants (handwriting sometimes makes distinguishing a capital K from a little k difficult).<br />
Example: N 2 (g) + 3 H 2 (g) 2 NH 3 (g)<br />
PNH3<br />
K eq =<br />
3<br />
P P<br />
N2<br />
2<br />
H2<br />
Thus, the equilibrium constant expression depends only on the reaction stoichiometry. For this<br />
reason, it is convention to write out the stoichiometry using only whole numbers. Using<br />
fractions can change the equilibrium constant. For example:<br />
[C]<br />
A + 2 B C K eq =<br />
2<br />
[A][B]