Ion-Selective Electrodes With Ionophore-Doped Sensing Membranes
Ion-Selective Electrodes With Ionophore-Doped Sensing Membranes
Ion-Selective Electrodes With Ionophore-Doped Sensing Membranes
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
2554 Supramolecular devices<br />
<strong>Ion</strong>-selective<br />
microelectrode<br />
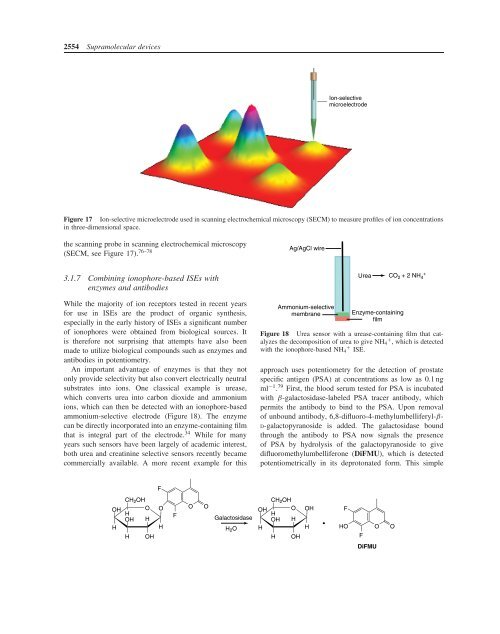
Figure 17 <strong>Ion</strong>-selective microelectrode used in scanning electrochemical microscopy (SECM) to measure profiles of ion concentrations<br />
in three-dimensional space.<br />
the scanning probe in scanning electrochemical microscopy<br />
(SECM, see Figure 17). 76–78<br />
Ag/AgCl wire<br />
3.1.7 Combining ionophore-based ISEs with<br />
enzymes and antibodies<br />
While the majority of ion receptors tested in recent years<br />
for use in ISEs are the product of organic synthesis,<br />
especially in the early history of ISEs a significant number<br />
of ionophores were obtained from biological sources. It<br />
is therefore not surprising that attempts have also been<br />
made to utilize biological compounds such as enzymes and<br />
antibodies in potentiometry.<br />
An important advantage of enzymes is that they not<br />
only provide selectivity but also convert electrically neutral<br />
substrates into ions. One classical example is urease,<br />
which converts urea into carbon dioxide and ammonium<br />
ions, which can then be detected with an ionophore-based<br />
ammonium-selective electrode (Figure 18). The enzyme<br />
can be directly incorporated into an enzyme-containing film<br />
that is integral part of the electrode. 34 While for many<br />
years such sensors have been largely of academic interest,<br />
both urea and creatinine selective sensors recently became<br />
commercially available. A more recent example for this<br />
Ammonium-selective<br />
membrane<br />
Urea CO 2 + 2 NH 4<br />
+<br />
Enzyme-containing<br />
film<br />
Figure 18 Urea sensor with a urease-containing film that catalyzes<br />
the decomposition of urea to give NH + 4 , which is detected<br />
with the ionophore-based NH + 4 ISE.<br />
approach uses potentiometry for the detection of prostate<br />
specific antigen (PSA) at concentrations as low as 0.1 ng<br />
ml −1 . 79 First, the blood serum tested for PSA is incubated<br />
with β-galactosidase-labeled PSA tracer antibody, which<br />
permits the antibody to bind to the PSA. Upon removal<br />
of unbound antibody, 6,8-difluoro-4-methylumbelliferyl-β-<br />
D-galactopyranoside is added. The galactosidase bound<br />
through the antibody to PSA now signals the presence<br />
of PSA by hydrolysis of the galactopyranoside to give<br />
difluoromethylumbelliferone (DiFMU), which is detected<br />
potentiometrically in its deprotonated form. This simple<br />
F<br />
OH<br />
H<br />
CH 2 OH<br />
O<br />
H<br />
OH H<br />
H OH<br />
O<br />
H<br />
F<br />
O<br />
O<br />
Galactosidase<br />
H 2 O<br />
OH<br />
H<br />
CH 2 OH<br />
O<br />
H<br />
OH H<br />
H OH<br />
OH<br />
H<br />
F<br />
HO<br />
F<br />
O<br />
O<br />
DiFMU