Ion-Selective Electrodes With Ionophore-Doped Sensing Membranes
Ion-Selective Electrodes With Ionophore-Doped Sensing Membranes
Ion-Selective Electrodes With Ionophore-Doped Sensing Membranes
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
2560 Supramolecular devices<br />
H 3 C<br />
C 7 H 15<br />
N<br />
O<br />
O<br />
H 3 C<br />
N<br />
C 7 H 15<br />
O<br />
O<br />
O<br />
O<br />
H 3 C<br />
N<br />
C 7 H 15<br />
O<br />
O<br />
H 3 C<br />
O<br />
O<br />
O<br />
O<br />
C 12 H 25<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
Na + -I<br />
Na + -II<br />
O<br />
O<br />
CH 2 CH 2<br />
O OC 2 H 5<br />
O OC 2 H 5<br />
O O<br />
CH 2<br />
CH 2<br />
O<br />
O<br />
O<br />
Na + -III<br />
Na + -IV<br />
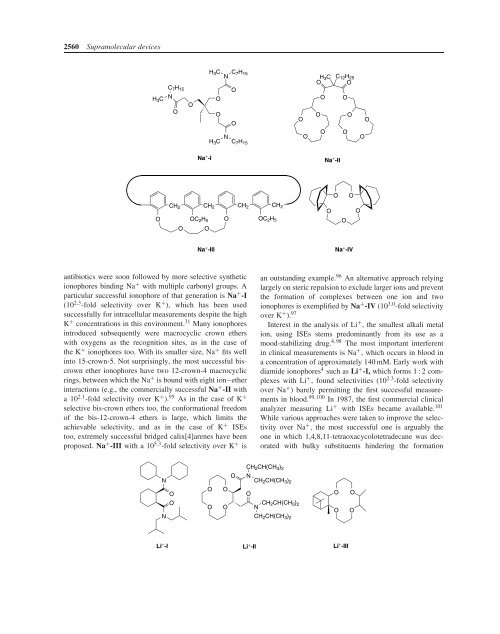
antibiotics were soon followed by more selective synthetic<br />
ionophores binding Na + with multiple carbonyl groups. A<br />
particular successful ionophore of that generation is Na + -I<br />
(10 2.3 -fold selectivity over K + ), which has been used<br />
successfully for intracellular measurements despite the high<br />
K + concentrations in this environment. 31 Many ionophores<br />
introduced subsequently were macrocyclic crown ethers<br />
with oxygens as the recognition sites, as in the case of<br />
the K + ionophores too. <strong>With</strong> its smaller size, Na + fits well<br />
into 15-crown-5. Not surprisingly, the most successful biscrown<br />
ether ionophores have two 12-crown-4 macrocyclic<br />
rings, between which the Na + is bound with eight ion–ether<br />
interactions (e.g., the commercially successful Na + -II with<br />
a10 2.1 -fold selectivity over K + ). 95 As in the case of K +<br />
selective bis-crown ethers too, the conformational freedom<br />
of the bis-12-crown-4 ethers is large, which limits the<br />
achievable selectivity, and as in the case of K + ISEs<br />
too, extremely successful bridged calix[4]arenes have been<br />
proposed. Na + -III with a 10 5.3 -fold selectivity over K + is<br />
an outstanding example. 96 An alternative approach relying<br />
largely on steric repulsion to exclude larger ions and prevent<br />
the formation of complexes between one ion and two<br />
ionophores is exemplified by Na + -IV (10 3.0 -fold selectivity<br />
over K + ). 97<br />
Interest in the analysis of Li + , the smallest alkali metal<br />
ion, using ISEs stems predominantly from its use as a<br />
mood-stabilizing drug. 4, 98 The most important interferent<br />
in clinical measurements is Na + , which occurs in blood in<br />
a concentration of approximately 140 mM. Early work with<br />
diamide ionophores 4 such as Li + -I, which forms 1 : 2 complexes<br />
with Li + , found selectivities (10 2.3 -fold selectivity<br />
over Na + ) barely permitting the first successful measurements<br />
in blood. 99, 100 In 1987, the first commercial clinical<br />
analyzer measuring Li + with ISEs became available. 101<br />
While various approaches were taken to improve the selectivity<br />
over Na + , the most successful one is arguably the<br />
one in which 1,4,8,11-tetraoxacycolotetradecane was decorated<br />
with bulky substituents hindering the formation<br />
N<br />
N<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
CH 2 CH(CH 3 ) 2<br />
N CH2 CH(CH 3 ) 2<br />
O<br />
CH 2 CH(CH 3 ) 2<br />
N<br />
CH 2 CH(CH 3 ) 2<br />
O<br />
O<br />
O<br />
O<br />
Li + -I<br />
Li + -II<br />
Li + -III