View Newsletter - Chemical Insight & Forecasting
View Newsletter - Chemical Insight & Forecasting
View Newsletter - Chemical Insight & Forecasting
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
CHEMICAL INDUSTRIES<br />
NEWSLETTER<br />
A monthly compilation of SRIC report abstracts and news<br />
January 2006<br />
CEH Marketing Research Report Abstract<br />
ETHYL ALCOHOL<br />
By<br />
Michael P. Malveda<br />
with<br />
Hossein Janshekar and Yoshio Inoguchi<br />
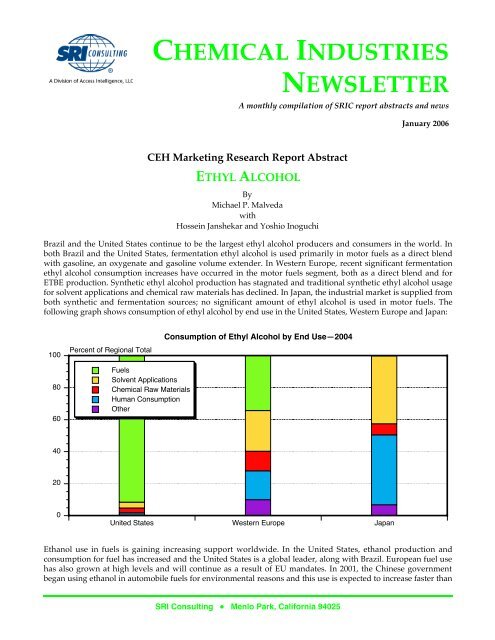
Brazil and the United States continue to be the largest ethyl alcohol producers and consumers in the world. In<br />
both Brazil and the United States, fermentation ethyl alcohol is used primarily in motor fuels as a direct blend<br />
with gasoline, an oxygenate and gasoline volume extender. In Western Europe, recent significant fermentation<br />
ethyl alcohol consumption increases have occurred in the motor fuels segment, both as a direct blend and for<br />
ETBE production. Synthetic ethyl alcohol production has stagnated and traditional synthetic ethyl alcohol usage<br />
for solvent applications and chemical raw materials has declined. In Japan, the industrial market is supplied from<br />
both synthetic and fermentation sources; no significant amount of ethyl alcohol is used in motor fuels. The<br />
following graph shows consumption of ethyl alcohol by end use in the United States, Western Europe and Japan:<br />
100<br />
Percent of Regional Total<br />
Consumption of Ethyl Alcohol by End Use—2004<br />
80<br />
60<br />
Fuels<br />
Solvent Applications<br />
<strong>Chemical</strong> Raw Materials<br />
Human Consumption<br />
Other<br />
40<br />
20<br />
0<br />
United States Western Europe Japan<br />
Ethanol use in fuels is gaining increasing support worldwide. In the United States, ethanol production and<br />
consumption for fuel has increased and the United States is a global leader, along with Brazil. European fuel use<br />
has also grown at high levels and will continue as a result of EU mandates. In 2001, the Chinese government<br />
began using ethanol in automobile fuels for environmental reasons and this use is expected to increase faster than<br />
SRI Consulting ● Menlo Park, California 94025
January 2006<br />
<strong>Chemical</strong> Industries <strong>Newsletter</strong><br />
any other ethanol use. Likewise, in India, the government decided to allow the sale of E-5 (5% ethyl alcohol blend<br />
in gasoline) in 2002. As a result, more ethanol plants are being constructed.<br />
Technological progress continues to increase production efficiency from feedstocks such as corn and sugar crops.<br />
Cost-effectiveness would aid producers and consumers alike. Continued research and development in cellulose<br />
production of ethanol would benefit the ethanol industry in the future.<br />
Legislation is a key factor worldwide for increasing and promoting ethanol fuel use. The new Energy Bill in the<br />
United States, the EU Biofuels Directive, the continued Caribbean Basin Initiative (CBI) tariff waivers, the<br />
privatization of ethanol industries, and promotion of and research into ethanol fuel use by some Asian<br />
governments all contribute to legislation and support for ethanol use. Ethanol’s use in solvent applications will<br />
continue to be affected by volatile organic compound (VOC) laws and regulations. This will limit ethanol use in<br />
this market segment.<br />
(For the complete marketing research report on ETHYL ALCOHOL, visit this report’s home page or see p. 644.5000 A of the <strong>Chemical</strong><br />
Economics Handbook.)<br />
CEH Marketing Research Report Abstract<br />
ETHYLENE-PROPYLENE ELASTOMERS<br />
By<br />
R. J. Chang<br />
with<br />
Masahiro Yoneyama<br />
Ethylene-propylene elastomers (EP elastomers) are the third-largest synthetic rubber consumed worldwide, after<br />
styrene-butadiene rubber and polybutadiene rubber. EP elastomers are characterized by their outstanding<br />
resistance to oxygen, ozone and heat, making them particularly useful in many applications such as automotive,<br />
roofing and power cables. Two basic types of EP elastomer are produced: ethylene-propylene copolymer (EPM),<br />
which requires vulcanization by means of free radical generators, and ethylene-propylene terpolymer (EPDM),<br />
made by copolymerizing ethylene and propylene with a small amount of a nonconjugated diolefin that is<br />
vulcanized in the conventional manner with sulfur or with peroxides. EPDM is estimated to account for 80–85%<br />
of total world production of ethylene-propylene elastomers. The following pie chart shows world consumption of<br />
EP elastomers in 2004:<br />
World Consumption of EP Elastomers—2004<br />
Other Asia<br />
India<br />
China<br />
Rest of<br />
the World<br />
North<br />
America<br />
Japan<br />
Eastern Europe<br />
Latin<br />
America<br />
Western<br />
Europe<br />
2 Visit us at www.sriconsulting.com
<strong>Chemical</strong> Industries <strong>Newsletter</strong> January 2006<br />
Supply was very tight in 2004 and some end users were put on allocation as consumption outstripped production<br />
slightly. It is believed that off-grade materials and changes in stocks accounted for the production deficit. Supply<br />
is expected to remain tight at least for the next two years because 2005 capacity includes 140 thousand metric tons<br />
of metallocene-based plants built by Dow and ExxonMobil, which are primarily for making ethylene/alpha-olefin<br />
copolymers. Although they can act as swing capacity for making metallocene-based EPDM, it is difficult or<br />
undesirable in practice. Thus, the effective operating rate for making EPDM is estimated at 91% in 2004–2005.<br />
Mitsui has announced plans to build a new metallocene-based plant with 75 thousand metric tons of capacity<br />
totally dedicated to production of EPDM, but the plant will not come on stream until 2007.<br />
(For the complete marketing research report on ETHYLENE-PROPYLENE ELASTOMERS, visit this report’s home page or see p. 525.2600 A<br />
of the <strong>Chemical</strong> Economics Handbook.)<br />
CEH Marketing Research Report Abstract<br />
HYDROGEN PEROXIDE<br />
By<br />
Stefan Schlag<br />
with<br />
Kazuteru Yokose<br />
Apparent consumption of hydrogen peroxide on a global basis is believed to have been almost 3 million metric<br />
tons in 2004. Consumption has increased substantially over the past few years, driven by global growth in the<br />
pulp and paper industry, in particular in China, Southeast Asia and South America. In South America, mining is<br />
a growing demand sector. In North America, consumption is projected to grow by about 1.8% and growth in<br />
Japan is forecast to be about 4.5%. Consumption will increase in Western Europe at an average annual rate of<br />
4.8% over 2004–2009, with the largest part of the additional consumption accounted for by the production of<br />
propylene oxide using the HPPO process.<br />
The following graph shows consumption of hydrogen peroxide by end use in North America, Western Europe<br />
and Japan:<br />
Consumption of Hydrogen Peroxide by End Use—2004<br />
100<br />
80<br />
60<br />
Percent of Regional Total<br />
Pulp and Paper<br />
<strong>Chemical</strong>s and Laundry Products<br />
Textiles<br />
Environmental Applications<br />
Mining<br />
Other<br />
40<br />
20<br />
0<br />
North America Western Europe Japan<br />
Visit us at www.sriconsulting.com 3
January 2006<br />
<strong>Chemical</strong> Industries <strong>Newsletter</strong><br />
The primary factor driving past and projected growth in hydrogen peroxide consumption is concern for the<br />
environment. Hydrogen peroxide, which decomposes into water and oxygen, is replacing oxidizing compounds<br />
such as chlorine in pulp bleaching and other applications. Hydrogen peroxide is also used as-is or in an activated<br />
form to treat inorganic and organic pollutants in industrial and municipal applications. While the hydrogen<br />
peroxide market has traditionally been diversified across a broad spectrum of uses, rapid growth in consumption<br />
in pulp bleaching has forced the business to be driven primarily by demand in this one application.<br />
Over the 2004–2009 period, hydrogen peroxide consumption will continue to be driven by demand in pulp<br />
bleaching and the new application of propylene oxide production, which is scheduled to start in 2008 and will<br />
consume an estimated 150 thousand metric tons by 2009. Demand in China, South America, Eastern Europe and<br />
Other Asia is projected to grow faster than in the developed regions.<br />
(For the complete marketing research report on HYDROGEN PEROXIDE, visit this report’s home page or see p. 741.5000 A of the <strong>Chemical</strong><br />
Economics Handbook.)<br />
CEH Marketing Research Report Abstract<br />
NATURAL FATTY ACIDS<br />
By<br />
Michael P. Malveda<br />
with<br />
Milen Blagoev, Ralf Gubler and Kazuo Yagi<br />
Many new fatty acid plants have been built in Southeast Asia, which is the major source of coconut, palm and<br />
palm kernel oils used as raw materials for C 8 -C 14 fatty acids. Altogether, producers of fatty acids from oil<br />
splitting in these countries (excluding China and India) have a total capacity of almost 2 million metric tons.<br />
Significant amounts of this increasing production are being exported to other world areas, including North<br />
America, Western Europe and Japan, all three of which are now net importers of fatty acids derived from fat and<br />
oil splitting.<br />
Worldwide production of fatty acids from splitting is dominated by two large producers—the Uniqema Group<br />
(formerly known as the Unichema Group and now owned by the Imperial <strong>Chemical</strong> Industries PLC) and the<br />
Cognis Group. The Uniqema Group operates four plants in different European countries, one plant in the United<br />
States and a joint venture in Malaysia, all with a total capacity of over 800 thousand metric tons. The Cognis<br />
Group operates one plant in the United States, one in Canada, one in Europe and a joint venture in Malaysia, with<br />
a total annual capacity of over 400 thousand metric tons. These capacity estimates exclude any fatty acid capacity<br />
that is part of the continuous soapmaking process and also exclude any capacity in Latin America or Eastern<br />
Europe. Indonesian producers Acidchem, Palm Oleo and Natural Oleochemicals also account for a significant<br />
amount of fatty acid splitting capacity. Another large producer is the Akzo Nobel <strong>Chemical</strong> Group, which<br />
operates plants in North America, Europe and Asia. In North America, Twin Rivers Technologies is a major<br />
producer.<br />
By far the largest worldwide tall oil fatty acid (TOFA) producer is the International Paper Company, which owns<br />
Arizona <strong>Chemical</strong> in the United States as well as TOFA producers in Finland, Sweden and the United Kingdom.<br />
It operates five plants.<br />
Global fatty acids supply continues to exceed fatty acids demand. In 2004, capacity utilization rates increased<br />
slightly in North America to about 85–90%. Industry sources believe that European capacity utilization is less<br />
(71%) than in North America. In Southeast Asia, capacity utilization rates are reported to be running at 80–90%.<br />
Asia currently accounts for 35% of global production and is expected to account for more than 50% of global fatty<br />
acid production by 2010.<br />
4 Visit us at www.sriconsulting.com
<strong>Chemical</strong> Industries <strong>Newsletter</strong> January 2006<br />
Whether used as such or in the form of various derivatives, fatty acids are ultimately consumed in a wide variety<br />
of end-use industries. The economic growth of many of these industries (e.g., rubber, plastics and detergents) is<br />
often a good indicator of the overall economic performance of a region. Not surprisingly, historical growth in the<br />
consumption of fatty acids has tended to approximate growth in regional GNP.<br />
(For the complete marketing research report on NATURAL FATTY ACIDS, visit this report’s home page or see p. 657.5000 A of the <strong>Chemical</strong><br />
Economics Handbook.)<br />
CEH Product Review Abstract<br />
POLYPHENYLENE SULFIDE RESINS<br />
By<br />
R. J. Chang<br />
with<br />
Thomas Kälin and Goro Toki<br />
Polyphenylene sulfide (PPS) resins exhibit exceptional resistance to thermal degradation and high heat distortion<br />
temperatures, as well as outstanding chemical and flame resistance. Major markets include automotive, electrical<br />
and electronics, industrial and appliances. World demand for PPS grew briskly at 9–11% per year during<br />
2001–2004, driven primarily by automotive applications. Although growth in the production of automobiles in<br />
North America, Western Europe and Japan has been sluggish, PPS resins have benefited from the ever-increasing<br />
under-the-hood temperatures of automobiles and the need for fuel and flame resistance in many automotive<br />
parts. This trend is expected to continue, and demand is projected to grow at approximately 10% per year during<br />
2004–2009.<br />
The following graph shows regional supply/demand for PPS resins as a percentage of the world total:<br />
World Supply/Demand for Polyphenylene Sulfide Resins—2004<br />
100<br />
Percent of World Total<br />
80<br />
60<br />
40<br />
20<br />
United States<br />
Western Europe<br />
Japan<br />
China<br />
Rest of the World<br />
0<br />
Annual Capacity Production Net Imports Net Exports Consumption<br />
In 2004, Japan and the United States remained the two dominant producing countries. In November 2005, supply<br />
was tight, and lead time in the United States was approximately twenty weeks. With world consumption<br />
Visit us at www.sriconsulting.com 5
January 2006<br />
<strong>Chemical</strong> Industries <strong>Newsletter</strong><br />
expected to grow at approximately 10% per year during 2004–2009, all producers announced plans to increase<br />
capacity.<br />
World capacity for PPS is expected to grow at an average annual rate of about 13% during 2004–2009.<br />
Consumption is forecast to grow at an average annual rate of 10%. China is expected to show the highest rates of<br />
growth in capacity and consumption (20% per year). The rest of the world (mostly other Asian countries) is also<br />
expected to show high growth rates of 20% per year. Consumption growth will be fueled mainly by automotive<br />
under-the-hood applications and by new extrusion and thermoforming grades that have been introduced into the<br />
market in the past two years.<br />
(For the complete product review on POLYPHENYLENE SULFIDE RESINS, visit this report’s home page or see p. 580.4600 A of the<br />
<strong>Chemical</strong> Economics Handbook.)<br />
PEP Report Abstract<br />
ADVANCES IN NAPHTHA STEAM CRACKING<br />
By<br />
Anthony Pavone<br />
(PEP Report 248A, December 2005)<br />
This PEP report is designed to help clients better understand the technology changes that are being incorporated<br />
in modern, state-of-the-art naphtha steam crackers, and also to assist operators of existing steam crackers in<br />
providing incremental upgrades to improve performance and profitability.<br />
For grass roots naphtha steam crackers, we provide an engineering process design and corresponding production<br />
economics for a 1.2 MM tpy naphtha steam cracking unit that incorporates state-of-the-art technology. These new<br />
developments include mercury and arsenic pretreatment of feedstock, DMSO addition to naphtha very low in<br />
sulfur content, furnace operation for both maximum ethylene and maximum propylene business objectives, front<br />
end depropanizer fractionation trains, dual depropanizer tower optimization, front end hydrogenation of<br />
diolefins with dilute hydrogen, mixed refrigerant cryogenics, and low pressure (20 bar) process compression. For<br />
this configuration, we have also provided material balances for light and full-range naphtha operated for both<br />
maximum ethylene and propylene yields.<br />
We have also prepared a comparison of the licensed process offerings currently made by KBR, Lummus, S&W,<br />
Technip and Linde.<br />
We also explore typical debottlenecking strategies to incrementally increase production capacity. These include<br />
cracked gas and refrigeration compressor upgrades, major driver modifications, quench oil and water wash tower<br />
modifications, and fractionation train tray and packing modifications.<br />
To extend major turnaround frequency to five-year intervals, we identify the major equipment that needs to be<br />
upgraded and/or made redundant. These include heavy service reboilers, compressor interstage coolers, TLE<br />
operation, upgraded furnace tube metallurgy, and quench cooler upgrades.<br />
Given the emphasis currently applied to on-line, real time economic optimizers, we review the vendor offerings,<br />
and describe the sources of economic credits that justify this type of SCADA investment.<br />
Lastly, we explore unit operating strategies to improve conversion and selectivity, reduce the volume of coke<br />
production and frequency of steam air decoking, and keep the unit running clean. We examine on-line washing of<br />
the cracked gas compressor, naphtha additives to reduce furnace tube coking and extend tube life, current<br />
hydrogenation catalyst availability, green oil management, intermediate draw-off trays on the primary<br />
fractionator for kerosene and diesel recovery, and improved process computer control offerings made by<br />
Honeywell and Foxboro.<br />
6 Visit us at www.sriconsulting.com
<strong>Chemical</strong> Industries <strong>Newsletter</strong> January 2006<br />
PEP Review Abstract<br />
BIODIESEL VIA AXENS HETEROGENEOUS CATALYSIS<br />
ESTERFIP-H PROCESS<br />
By<br />
Ronald G. Bray<br />
(PEP Review 2005-07, December 2005)<br />
Biodiesel is the methyl ester of fatty acids derived from renewable resources such as virgin vegetable oil, animal<br />
fats and used cooking oil. This biofuel can be used as a replacement for petroleum-based diesel in compression<br />
ignition engines with minimal modifications.<br />
The annual rate of growth in consumption of biodiesel during 1999–2004 exceeded 32%, reaching a global level of<br />
4.84 million tons/yr in 2004 (<strong>Chemical</strong> Economics Handbook estimates). Expansion is projected to continue at this<br />
phenomenal rate as a result of a variety of factors, including concerns about energy security, the environment and<br />
the skyrocketing cost of petroleum.<br />
The consumption of this biofuel is encouraged through various governmental incentives. EU Directive<br />
2003/30/EC set targets for a minimum fuels content from renewable resources with the goal of increasing content<br />
from today’s
January 2006<br />
CEH REPORTS AND PRODUCT<br />
REVIEWS IN PREPARATION<br />
Report Title<br />
Alkyl Acetates<br />
Amino Acids<br />
Calcium Chloride<br />
Cellulose Acetate Flake<br />
Cellulose Acetate/Triacetate Fibers<br />
Chlorobenzenes<br />
C 2 Chlorinated Solvents<br />
Ethanolamines<br />
Ethylbenzene<br />
Gasoline Octane Improvers<br />
Hydrochloric Acid<br />
Isophthalic Acid<br />
Isopropanolamines<br />
Lime/Limestone<br />
Neopentyl Polyhydric Alcohols<br />
Oxo <strong>Chemical</strong>s<br />
Phosphate Rock<br />
Plasticizer Alcohols<br />
Plasticizers<br />
Polyamide Resins (Nonnylon)<br />
Polyester Polyols<br />
Polyether Polyols for Urethanes<br />
Polyurethane Elastomers<br />
Polyurethane Foams<br />
Propylene Oxide<br />
Sodium Bicarbonate<br />
Sodium Carbonate<br />
Sodium Cyanide<br />
Styrene<br />
Styrenic Copolymers<br />
Sulfur<br />
Author<br />
Sebastian Bizzari<br />
Mike Malveda<br />
Stefan Schlag<br />
Stefan Müller<br />
Stefan Müller<br />
Thomas Kälin<br />
Jamie Lacson<br />
Elvira Greiner<br />
Koon-Ling Ring<br />
Michael Malveda<br />
Jamie Lacson<br />
Koon-Ling Ring<br />
Elvira Greiner<br />
Stefan Schlag<br />
Sebastian Bizzari<br />
Sebastian Bizzari<br />
Bala Suresh<br />
Sebastian Bizzari<br />
Sebastian Bizzari<br />
Elvira Greiner<br />
Henry Chinn<br />
Henry Chinn<br />
Henry Chinn<br />
Henry Chinn<br />
Henry Chinn<br />
Stefan Schlag<br />
Stefan Schlag<br />
Bala Suresh<br />
Koon-Ling Ring<br />
Koon-Ling Ring<br />
Bala Suresh<br />
This list is provided for the benefit of <strong>Chemical</strong> Economics<br />
Handbook users who may simultaneously be undertaking their<br />
own studies in these areas. Clients are welcome to write or call<br />
us in order to discuss the work in progress.<br />
CEH REPORTS AVAILABLE SEPARATELY<br />
To obtain a list of CEH marketing research reports or product<br />
reviews for sale separately, please see our website at<br />
http://www.sriconsulting.com/CEH/Public/Reports/ or<br />
contact:<br />
Ralf Gubler, Director, or<br />
Sebastian Bizzari/Koon-Ling Ring, Assistant<br />
Directors<br />
<strong>Chemical</strong> Economics Handbook Program<br />
SRI Consulting<br />
4300 Bohannon Drive, Suite 200<br />
Menlo Park, CA 94025<br />
Tel. (650) 384-4300 Fax: (650) 330-1149<br />
<strong>Chemical</strong> Industries <strong>Newsletter</strong><br />
NEW, Expanded<br />
Chemweek’s Business Daily<br />
Try 10 issues for FREE—your daily<br />
“executive briefing” of chemical industry<br />
news, insight and intelligence.<br />
As an SRIC client,<br />
you are eligible for<br />
a special free trial<br />
subscription to<br />
Chemweek’s<br />
Business Daily,<br />
the newly enhanced<br />
publication that<br />
delivers an<br />
executive briefing of<br />
the latest and most<br />
relevant chemical<br />
industry news—<br />
right to your<br />
desktop.<br />
Chemweek’s Business Daily is the chemical<br />
industry’s premiere source for news, insight and<br />
intelligence covering the global chemical and allied<br />
industries. You get a daily executive briefing on:<br />
Strategic moves, M&A activity, and<br />
financial plays<br />
Market and pricing movements for a<br />
growing list of 20 chemical categories<br />
Global regulatory and security issues<br />
New research, technologies and services<br />
that can provide a quantum shift in the<br />
global chemical business<br />
Key developments in petrochemical news,<br />
especially regarding pricing<br />
Special features include individual product focus<br />
reports and statistics on leading indicators,<br />
exclusive reports on specialty and fine chemicals,<br />
the latest in construction projects, price reports on<br />
basic chemicals and the CW 75 Stock Index with<br />
analysis and commentary.<br />
So, take advantage of this offer and see why<br />
readers consider this the daily must-read<br />
publication in the chemicals marketplace.<br />
To get your free 10 issues, click here<br />
http://www.chemweek.com/daily/<br />
and key in GBO335 when prompted.<br />
ACT NOW to take advantage<br />
of this special offer.<br />
8 Visit us at www.sriconsulting.com
<strong>Chemical</strong> Industries <strong>Newsletter</strong> January 2006<br />
CHEMICAL INDUSTRIES NEWSLETTER<br />
The <strong>Chemical</strong> Industries <strong>Newsletter</strong> is published monthly by SRI Consulting. The contents of the <strong>Newsletter</strong> are drawn from current research<br />
and publications of SRIC’s multiclient programs. Readers are welcome to call or write for more information about the subjects and programs<br />
mentioned (see addresses and telephone/fax numbers below).<br />
SRI Consulting offers the world’s most comprehensive ongoing multiclient databases on the chemical industry. The major multiclient programs<br />
include<br />
The ASEAN Report<br />
Directory of <strong>Chemical</strong> Producers<br />
<strong>Chemical</strong> Economics Handbook Canada Mexico<br />
The China Report China Middle East<br />
Process Economics East Asia South/Central America<br />
Specialty <strong>Chemical</strong>s Europe United States<br />
World Petrochemicals<br />
India<br />
Companies may participate in these continuing programs for the chemical industry through annual subscriptions or by purchasing individual<br />
reports. Each program is supported by inquiry and consulting privileges; electronic access is also available for all of these products.<br />
SRI Consulting................................................................John Pearson, President and CEO<br />
George Intille, Vice President<br />
<strong>Chemical</strong> Economics Handbook................................................Ralf Gubler, Director<br />
Directory of <strong>Chemical</strong> Producers...........................................Carolyn Read, Director<br />
Process Economics .................................................Russell Heinen, Group Director<br />
Production/Databases..........................................................Steven F. Read, Director<br />
Specialty <strong>Chemical</strong>s Update Program..................................Phil Calderoni, Director<br />
World Petrochemicals ..............................................Russell Heinen, Group Director<br />
About SRI Consulting<br />
SRI Consulting provides the world’s most comprehensive ongoing databases on the chemical industries. We offer an array of research-based<br />
programs designed to provide clients with specific market intelligence and analysis. These programs, combined with strategic information<br />
services, help clients define new market opportunities, identify and communicate future challenges, formulate and implement business<br />
strategies, and develop innovative products, processes and services. SRIC provides creative yet practical strategies, supported by renowned<br />
industry and technology expertise and delivered by multidisciplinary teams working closely with clients to ensure implementation. SRI<br />
Consulting is a division of Access Intelligence, LLC.<br />
About Access Intelligence, LLC<br />
Access Intelligence, LLC is a full-service global information and marketing solutions provider of competitive business-to-business information.<br />
The company publishes daily news services, premium-value newsletters, subscription-based websites, magazines, directories, and<br />
databases.<br />
SRI Consulting<br />
Headquarters<br />
_________________________ International Offices _________________________<br />
Menlo Park, CA Europe, Middle East and Africa Beijing<br />
John Pearson, President and CEO Toedistrasse 23, 1st Floor COFCO Plaza<br />
4300 Bohannon Drive, Suite 200 8002 Zürich, Switzerland Suite 420–421, Tower B<br />
Menlo Park, CA 94025 Telephone: 41 44 283 63 33 No. 8 Jianguomennei Avenue<br />
Telephone: (650) 384-4300 Fax: 41 44 283 63 30 Beijing, China 100005<br />
Fax: (650) 330-1149 zürich@sriconsulting.com Telephone: 86 10 6526 0698<br />
menlopark@sriconsulting.com Fax: 86 10 6526 0934<br />
beijing@sriconsulting.com<br />
U.S. Offices<br />
East Asia<br />
Houston<br />
Takeda Honcho Building, 8th Floor<br />
16945 Northchase Drive 2-1-7 Nihonbashi Honcho<br />
4 Greenspoint Plaza, Suite 1910 Chuoku, Tokyo 103-0023, Japan<br />
Houston, TX 77060 Telephone: 81 3 5202-7320<br />
Telephone: (281) 876-6923 Fax: 81 3 5202-7333<br />
Fax: (281) 876-6947 tokyo@sriconsulting.com<br />
houston@sriconsulting.com<br />
<strong>Chemical</strong> Industries <strong>Newsletter</strong><br />
Ellen Blue, Editor<br />
© 2006 by SRI Consulting.<br />
All rights reserved. Unauthorized reproduction prohibited.<br />
Visit us at www.sriconsulting.com 9