Round 1 - Foundation for Innovative New Diagnostics
Round 1 - Foundation for Innovative New Diagnostics
Round 1 - Foundation for Innovative New Diagnostics
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
10.3. Phase 2 - Wild type P. falciparum<br />
and P. vivax and Plasmodium spp.<br />
negative samples<br />
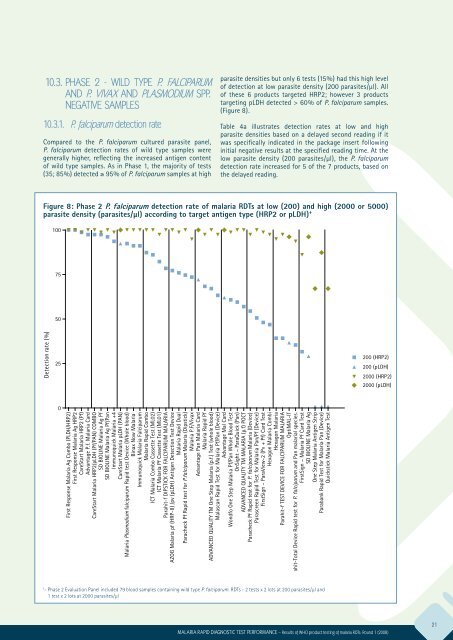
10.3.1. P. falciparum detection rate<br />
Compared to the P. falciparum cultured parasite panel,<br />
P. falciparum detection rates of wild type samples were<br />
generally higher, reflecting the increased antigen content<br />
of wild type samples. As in Phase 1, the majority of tests<br />
(35; 85%) detected ≥ 95% of P. falciparum samples at high<br />
parasite densities but only 6 tests (15%) had this high level<br />
of detection at low parasite density (200 parasites/μl). All<br />
of these 6 products targeted HRP2; however 3 products<br />
targeting pLDH detected > 60% of P. falciparum samples.<br />
(Figure 8).<br />
Table 4a illustrates detection rates at low and high<br />
parasite densities based on a delayed second reading if it<br />
was specifically indicated in the package insert following<br />
initial negative results at the specified reading time. At the<br />
low parasite density (200 parasites/μl), the P. falciparum<br />
detection rate increased <strong>for</strong> 5 of the 7 products, based on<br />
the delayed reading.<br />
Figure 8: Phase 2 P. falciparum detection rate of malaria RDTs at low (200) and high (2000 or 5000)<br />
parasite density (parasites/μl) according to target antigen type (HRP2 or pLDH) †<br />
100<br />
75<br />
50<br />
Detection rate (%)<br />
25<br />
200 (HRP2)<br />
200 (pLDH)<br />
2000 (HRP2)<br />
2000 (pLDH)<br />
0<br />
First Response Malaria Ag Combo (PLDH/HRP2)<br />
First Response Malaria Ag HRP2<br />
CareStart Malaria HRP2 (Pf)<br />
Advantage P.f. Malaria Card<br />
CareStart Malaria HRP2/pLDH (Pf/PAN) COMBO<br />
SD BIOLINE Malaria Ag Pf<br />
SD BIOLINE Malaria Ag Pf/Pan<br />
Immunoquick Malaria +4<br />
CareStart Malaria pLDH (PAN)<br />
Malaria Plasmodium falciparum Rapid test Device (Whole blood)<br />
Binax Now Malaria<br />
Immunoquick Malaria Falciparum<br />
Malaria Rapid Combo<br />
ICT Malaria Combo Cassette Test (ML02)<br />
ICT Malaria Pf Cassette Test (ML01)<br />
Parahit-f DIPSTICK FOR FALCIPARUM MALARIA<br />
AZOG Malaria pf (HRP-II) /pv (pLDH) Antigen Detection Test Device<br />
Malaria Rapid Dual<br />
Paracheck Pf Rapid test <strong>for</strong> P.falciparum Malaria (Dipstick)<br />
Malaria P.F/Vivax<br />
Advantage Pan Malaria Card<br />
Malaria Rapid Pf<br />
ADVANCED QUALITY TM One Step Malaria (p.f.) Test (whole blood)<br />
Malascan Rapid Test <strong>for</strong> Malaria Pf/Pan (Device)<br />
Advantage Mal Card<br />
Wondfo One Step Malaria Pf/Pan Whole Blood Test<br />
OnSight – ParaQuick (Pan)<br />
ADVANCED QUALITY TM MALARIA (p.f) POCT<br />
Paracheck Pf Rapid test <strong>for</strong> P. falciparum Malaria (Device)<br />
Parascreen Rapid Test <strong>for</strong> Malaria Pan/Pf (Device)<br />
FirstSign – ParaView-2 (Pv + Pf) Card Test<br />
Hexagon Malaria Combi<br />
Hexagon Malaria<br />
Parahit-f TEST DEVICE FOR FALCIPARUM MALARIA<br />
OptiMAL-IT<br />
Parahit-Total Device Rapid test <strong>for</strong> P. falciparum and Pan malarial species.<br />
FirstSign – Malaria Pf Card Test<br />
SD BIOLINE Malaria Ag<br />
One Step Malaria Antigen Strip<br />
Parabank Rapid Test <strong>for</strong> Malaria Pan (Device)<br />
Quickstick Malaria Antigen Test<br />
†<br />
- Phase 2 Evaluation Panel included 79 blood samples containing wild type P. falciparum. RDTs - 2 tests x 2 lots at 200 parasites/μl and<br />
1 test x 2 lots at 2000 parasites/μl<br />
Malaria Rapid Diagnostic Test Per<strong>for</strong>mance – Results of WHO product testing of malaria RDTs: <strong>Round</strong> 1 (2008)<br />
21






![Download in English [pdf 2Mb] - Foundation for Innovative New ...](https://img.yumpu.com/49580359/1/184x260/download-in-english-pdf-2mb-foundation-for-innovative-new-.jpg?quality=85)






![New laboratory diagnostic tools for tuberculosis control [.pdf]](https://img.yumpu.com/43339906/1/190x135/new-laboratory-diagnostic-tools-for-tuberculosis-control-pdf.jpg?quality=85)

