Round 1 - Foundation for Innovative New Diagnostics
Round 1 - Foundation for Innovative New Diagnostics
Round 1 - Foundation for Innovative New Diagnostics
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
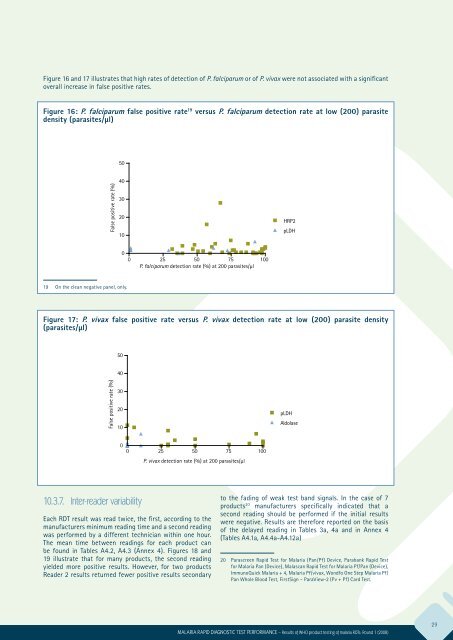
Figure 16 and 17 illustrates that high rates of detection of P. falciparum or of P. vivax were not associated with a significant<br />
overall increase in false positive rates.<br />
Figure 16: P. falciparum false positive rate 19 versus P. falciparum detection rate at low (200) parasite<br />
density (parasites/μl)<br />
50<br />
False positive rate (%)<br />
40<br />
30<br />
20<br />
10<br />
HRP2<br />
pLDH<br />
19<br />
0<br />
0 25 50 75 100<br />
P. falciparum detection rate (%) at 200 parasites/µl<br />
19 On the clean negative panel, only.<br />
Figure 17: P. vivax false positive rate versus P. vivax detection rate at low (200) parasite density<br />
(parasites/μl)<br />
50<br />
40<br />
False positive rate (%)<br />
30<br />
20<br />
10<br />
pLDH<br />
Aldolase<br />
0<br />
0 25 50 75 100<br />
P. vivax detection rate (%) at 200 parasites/µl<br />
10.3.7. Inter-reader variability<br />
Each RDT result was read twice, the first, according to the<br />
manufacturers minimum reading time and a second reading<br />
was per<strong>for</strong>med by a different technician within one hour.<br />
The mean time between readings <strong>for</strong> each product can<br />
be found in Tables A4.2, A4.3 (Annex 4). Figures 18 and<br />
19 illustrate that <strong>for</strong> many products, the second reading<br />
yielded more positive results. However, <strong>for</strong> two products<br />
Reader 2 results returned fewer positive results secondary<br />
to the fading of weak test band signals. In the case of 7<br />
products 20 manufacturers specifically indicated that a<br />
second reading should be per<strong>for</strong>med if the initial results<br />
were negative. Results are there<strong>for</strong>e reported on the basis<br />
of the delayed reading in Tables 3a, 4a and in Annex 4<br />
(Tables A4.1a, A4.4a-A4.12a)<br />
20 Parascreen Rapid Test <strong>for</strong> Malaria (Pan/Pf) Device, Parabank Rapid Test<br />
<strong>for</strong> Malaria Pan (Device), Malascan Rapid Test <strong>for</strong> Malaria Pf/Pan (Device),<br />
ImmunoQuick Malaria + 4, Malaria Pf/vivax, Wondfo One Step Malaria Pf/<br />
Pan Whole Blood Test, FirstSign – ParaView-2 (Pv + Pf) Card Test.<br />
Malaria Rapid Diagnostic Test Per<strong>for</strong>mance – Results of WHO product testing of malaria RDTs: <strong>Round</strong> 1 (2008)<br />
29






![Download in English [pdf 2Mb] - Foundation for Innovative New ...](https://img.yumpu.com/49580359/1/184x260/download-in-english-pdf-2mb-foundation-for-innovative-new-.jpg?quality=85)






![New laboratory diagnostic tools for tuberculosis control [.pdf]](https://img.yumpu.com/43339906/1/190x135/new-laboratory-diagnostic-tools-for-tuberculosis-control-pdf.jpg?quality=85)

