Round 1 - Foundation for Innovative New Diagnostics
Round 1 - Foundation for Innovative New Diagnostics
Round 1 - Foundation for Innovative New Diagnostics
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
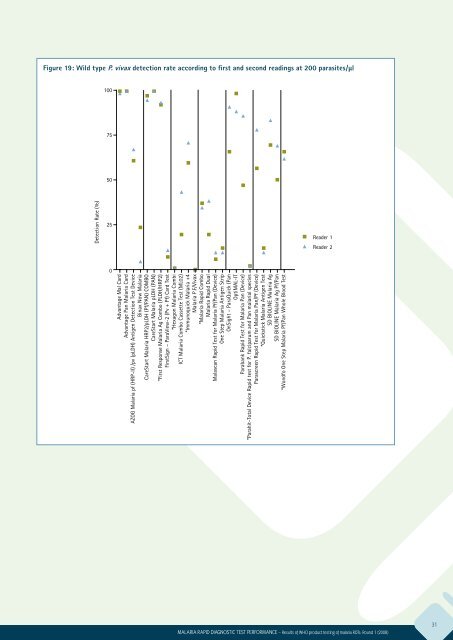
Figure 19: Wild type P. vivax detection rate according to first and second readings at 200 parasites/μl<br />
100<br />
75<br />
50<br />
Detection Rate (%)<br />
25<br />
Reader 1<br />
Reader 2<br />
0<br />
Advantage Mal Card<br />
Advantage Pan Malaria Card<br />
AZOG Malaria pf (HRP-II) /pv (pLDH) Antigen Detection Test Device<br />
Binax Now Malaria<br />
CareStart Malaria HRP2/pLDH (Pf/PAN) COMBO<br />
CareStart Malaria pLDH (PAN)<br />
*First Response Malaria Ag Combo (PLDH/HRP2)<br />
FirstSign – ParaView-2 (Pv + Pf) Card Test<br />
*Hexagon Malaria Combi<br />
ICT Malaria Combo Cassette Test (ML02)<br />
*Immunoquick Malaria +4<br />
Malaria P.F/Vivax<br />
*Malaria Rapid Combo<br />
Malaria Rapid Dual<br />
Malascan Rapid Test <strong>for</strong> Malaria Pf/Pan (Device)<br />
One Step Malaria Antigen Strip<br />
OnSight – ParaQuick (Pan<br />
OptiMAL-IT<br />
Parabank Rapid Test <strong>for</strong> Malaria Pan (Device)<br />
*Parahit-Total Device Rapid test <strong>for</strong> P. falciparum and Pan malarial species<br />
Parascreen Rapid Test <strong>for</strong> Malaria Pan/Pf (Device)<br />
*Quickstick Malaria Antigen Test<br />
SD BIOLINE Malaria Ag<br />
SD BIOLINE Malaria Ag Pf/Pan<br />
*Wondfo One Step Malaria Pf/Pan Whole Blood Test<br />
Malaria Rapid Diagnostic Test Per<strong>for</strong>mance – Results of WHO product testing of malaria RDTs: <strong>Round</strong> 1 (2008)<br />
31






![Download in English [pdf 2Mb] - Foundation for Innovative New ...](https://img.yumpu.com/49580359/1/184x260/download-in-english-pdf-2mb-foundation-for-innovative-new-.jpg?quality=85)






![New laboratory diagnostic tools for tuberculosis control [.pdf]](https://img.yumpu.com/43339906/1/190x135/new-laboratory-diagnostic-tools-for-tuberculosis-control-pdf.jpg?quality=85)

