Round 1 - Foundation for Innovative New Diagnostics
Round 1 - Foundation for Innovative New Diagnostics
Round 1 - Foundation for Innovative New Diagnostics
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
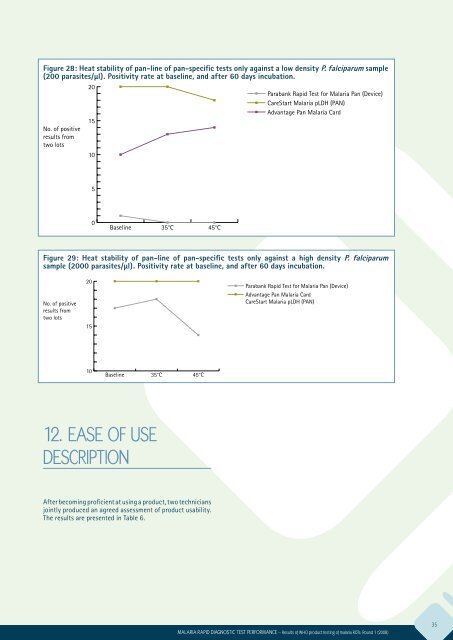
Figure 28: Heat stability of pan-line of pan-specific tests only against a low density P. falciparum sample<br />
(200 parasites/μl). Positivity rate at baseline, and after 60 days incubation.<br />
No. of positive<br />
results from<br />
two lots<br />
20<br />
15<br />
10<br />
Parabank Rapid Test <strong>for</strong> Malaria Pan (Device)<br />
CareStart Malaria pLDH (PAN)<br />
Advantage Pan Malaria Card<br />
5<br />
0<br />
Baseline<br />
35°C<br />
45°C<br />
Figure 29: Heat stability of pan-line of pan-specific tests only against a high density P. falciparum<br />
sample (2000 parasites/μl). Positivity rate at baseline, and after 60 days incubation.<br />
No. of positive<br />
results from<br />
two lots<br />
20<br />
15<br />
Parabank Rapid Test <strong>for</strong> Malaria Pan (Device)<br />
Advantage Pan Malaria Card<br />
CareStart Malaria pLDH (PAN)<br />
10<br />
Baseline<br />
35°C<br />
45°C<br />
12. Ease of use<br />
description<br />
After becoming proficient at using a product, two technicians<br />
jointly produced an agreed assessment of product usability.<br />
The results are presented in Table 6.<br />
Malaria Rapid Diagnostic Test Per<strong>for</strong>mance – Results of WHO product testing of malaria RDTs: <strong>Round</strong> 1 (2008)<br />
35






![Download in English [pdf 2Mb] - Foundation for Innovative New ...](https://img.yumpu.com/49580359/1/184x260/download-in-english-pdf-2mb-foundation-for-innovative-new-.jpg?quality=85)






![New laboratory diagnostic tools for tuberculosis control [.pdf]](https://img.yumpu.com/43339906/1/190x135/new-laboratory-diagnostic-tools-for-tuberculosis-control-pdf.jpg?quality=85)

