basal cell carcinoma update - The Dermatologist
basal cell carcinoma update - The Dermatologist
basal cell carcinoma update - The Dermatologist
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
BASAL CELL CARCINOMA<br />
UPDATE: RECENT RESEARCH<br />
AND EMERGING TREATMENT<br />
OPTIONS<br />
Online<br />
Exclusive<br />
Two recent articles in the New England Journal of Medicine about vismodegib provide additional evidence for the use of the drug in the treatment of advanced BCC and new evidence for the<br />
use of the drug in patients with <strong>basal</strong> <strong>cell</strong> nevus syndrome, and a new study from researchers in Boston further confirms that caffeine may help reduce an individual’s risk of developing BCC.<br />
JULIA ERNST, MS, ASSISTANT EDITOR<br />
developments for BCC. <strong>The</strong> efficacy of<br />
vismodegib has been further supported<br />
with the results of a recent study<br />
in the New England Journal of Medicine<br />
(NEJM), and another article in the<br />
same issue of NEJM demonstrates that<br />
the drug is also effective for patients<br />
with the rare, heritable disease <strong>basal</strong><br />
<strong>cell</strong> nevus syndrome (BCNS), which<br />
is sometimes referred to as Gorlin syndrome.<br />
3,4 In addition, researchers have<br />
demonstrated that caffeine consumption<br />
may lower an individual’s risk for<br />
developing BCC. 5,6,7<br />
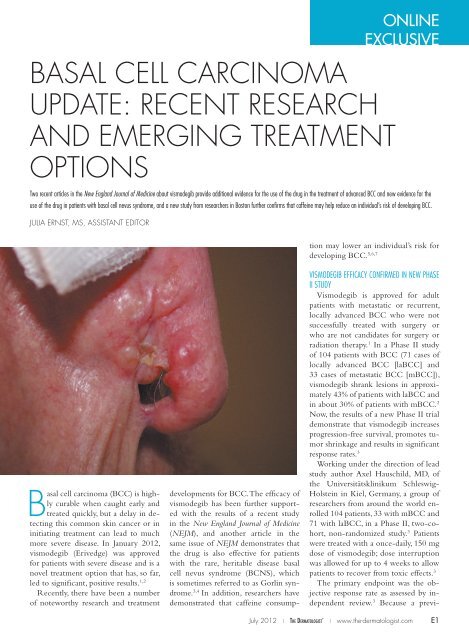
Basal <strong>cell</strong> <strong>carcinoma</strong> (BCC) is highly<br />
curable when caught early and<br />
treated quickly, but a delay in detecting<br />
this common skin cancer or in<br />
initiating treatment can lead to much<br />
more severe disease. In January 2012,<br />
vismodegib (Erivedge) was approved<br />
for patients with severe disease and is a<br />
novel treatment option that has, so far,<br />
led to significant, positive results. 1,2<br />
Recently, there have been a number<br />
of noteworthy research and treatment<br />
VISMODEGIB EFFICACY CONFIRMED IN NEW PHASE<br />
II STUDY<br />
Vismodegib is approved for adult<br />
patients with metastatic or recurrent,<br />
locally advanced BCC who were not<br />
successfully treated with surgery or<br />
who are not candidates for surgery or<br />
radiation therapy. 1 In a Phase II study<br />
of 104 patients with BCC (71 cases of<br />
locally advanced BCC [laBCC] and<br />
33 cases of metastatic BCC [mBCC]),<br />
vismodegib shrank lesions in approximately<br />
43% of patients with laBCC and<br />
in about 30% of patients with mBCC. 2<br />
Now, the results of a new Phase II trial<br />
demonstrate that vismodegib increases<br />
progression-free survival, promotes tumor<br />
shrinkage and results in significant<br />
response rates. 3<br />
Working under the direction of lead<br />
study author Axel Hauschild, MD, of<br />
the Universitätsklinikum Schleswig-<br />
Holstein in Kiel, Germany, a group of<br />
researchers from around the world enrolled<br />
104 patients, 33 with mBCC and<br />
71 with laBCC, in a Phase II, two-cohort,<br />
non-randomized study. 3 Patients<br />
were treated with a once-daily, 150 mg<br />
dose of vismodegib; dose interruption<br />
was allowed for up to 4 weeks to allow<br />
patients to recover from toxic effects. 3<br />
<strong>The</strong> primary endpoint was the objective<br />
response rate as assessed by independent<br />
review. 3 Because a previ-<br />
July 2012 | <strong>The</strong> <strong>Dermatologist</strong> ® | www.the-dermatologist.com E1
Online Exclusive<br />
ously defined standard endpoint for<br />
laBCC did not exist when the study<br />
was designed, the researchers defined<br />
response “as a decrease of 30% or more<br />
in the externally visible or radiographic<br />
dimension (if applicable) or complete<br />
resolution of ulceration (if present at<br />
baseline).” 3 Progressive disease was defined<br />
as “an increase of 20% or more<br />
in the externally visible or radiographic<br />
dimensions (if applicable), new ulceration<br />
or a new lesion.” 3<br />
Patients with mBCC all had partial<br />
responses; according to the researchers,<br />
the majority of these patients (24/33, or<br />
73%) had tumor shrinkage. 3 <strong>The</strong> median<br />
duration of objective response in these<br />
mBCC patients was 7.6 months (range,<br />
2.1 to 11.1), according to independent<br />
review, and investigator-determined response<br />
duration was 12.9 months (range,<br />
1.9 to 12.9). 3 Median progression-free<br />
survival was 9.5 months (95% confidence<br />
interval [CI], 7.4 to not estimable),<br />
according to independent review,<br />
and 9.2 months (95% CI, 7.4 to not estimable),<br />
according to the estimates of the<br />
site investigators. 3 Overall survival data<br />
were not mature at the time the study<br />
was published. 3<br />
Patients with laBCC had an objective<br />
response rate of 43%. 3 A complete<br />
response, defined as the absence of residual<br />
BCC on assessment of a biopsy<br />
specimen, was observed in 13 patients<br />
(21%). 3 <strong>The</strong> median duration of response<br />
was 7.6 months (range, 1.0 to<br />
12.9 months), according to independent<br />
review, and 7.6 months (range, 1.4<br />
to 16.6 months), according to the site<br />
investigators. 3 Biopsy specimens from<br />
54% of patients in this cohort showed<br />
no residual BCC, and visible reductions<br />
in tumor size and improvement in appearance<br />
were noted by site investigators<br />
for the majority of these patients. 3<br />
According to the researchers, all patients<br />
experienced at least one adverse<br />
event (AE) during the study; more than<br />
half of the treated patients (57%) had<br />
only Grade 1 or 2 events. 3 Grade 3 and<br />
4 AEs included muscle spasms, weight<br />
loss, fatigue and loss of appetite. 3 Of<br />
the 104 total patients, 13 (12%) experienced<br />
an AE that led to discontinuation<br />
of vismodegib. 3<br />
Serious AEs were reported in 26<br />
patients (25%). 3 Fatal adverse events<br />
(Grade 5) were reported in seven patients<br />
(one with mBCC and six with<br />
laBCC): death from an unknown cause<br />
(in three patients) and hypovolemic<br />
shock, myocardial infarction, meningeal<br />
disease and ischemic stroke (in one<br />
patient each). 3<br />
According to Ervin Epstein, MD, of<br />
Children’s Hospital Oakland Research<br />
Institute (CHORI) in Oakland, CA,<br />
the results of this worldwide study show<br />
“significant clinical benefit for the use<br />
of the drug in patients with locally advanced<br />
and metastatic disease.” Dr. Epstein’s<br />
research focuses on non-melanoma<br />
skin cancer, particularly BCC.<br />
VISMODEGIB DEMONSTRATES POSITIVE INITIAL<br />
RESULTS FOR PATIENTS WITH BASAL CELL NEVUS<br />
SYNDROME<br />
BCNS is a rare, heritable condition<br />
that can lead to the development of<br />
hundreds to thousands of BCCs in a<br />
single patient. 4 Individuals with BCNS<br />
are also at an increased risk for meduloblastomas<br />
and rhabdomyosarcomas. 4<br />
Currently, there is no pharmacologic<br />
therapy for patients with BCNS, but<br />
the second article published in NEJM<br />
reports that vismodegib may also be effective<br />
for patients with the syndrome. 4<br />
<strong>The</strong> Phase II trial enrolled 42 patients<br />
with BCNS, all of whom had at<br />
least 10 surgically eligible BCCs present<br />
at study entry or removed during<br />
the previous 2 years. 4 Participants were<br />
randomized to receive either oral vismodegib<br />
at a dose of 150 mg daily or<br />
placebo for 18 months. 4<br />
<strong>The</strong> researchers tracked more than<br />
2,000 existing and 694 new, surgically<br />
eligible BCCs. 4 Vismodegib was found<br />
to significantly reduce “the per-patient<br />
rate of new surgically eligible <strong>basal</strong><br />
<strong>cell</strong> <strong>carcinoma</strong>s below that of the placebo<br />
group (mean, 2 vs. 29 new surgically<br />
eligible <strong>basal</strong> <strong>cell</strong> <strong>carcinoma</strong>s per<br />
year; median, 2 vs. 25 new surgically<br />
eligible <strong>basal</strong> <strong>cell</strong> <strong>carcinoma</strong>s per year;<br />
P
Online Exclusive<br />
Erin Gilbert, MD, PhD, an assistant<br />
professor of dermatology at<br />
SUNY Downstate Medical Center in<br />
New York, NY, who is in practice at<br />
Gramercy Park Dermatology in New<br />
York, concurs.<br />
“Ongoing treatment with vismodegib<br />
holds promise for those patients<br />
with <strong>basal</strong> <strong>cell</strong> nevus syndrome who<br />
can tolerate the medication,” Dr. Gilbert<br />
explains. “Our primary concern<br />
is about longer term exposures to the<br />
drug and about the burden of cancer<br />
when and if their treatment is stopped.”<br />
While the drug did lead to side effects,<br />
according to Dr. Epstein, including<br />
leg cramps and hair and weight<br />
loss, the AEs reported in patients with<br />
BCNS were similar to those experienced<br />
by patients receiving vismodegib<br />
in other trials. 4<br />
Dr. Epstein is conducting ongoing<br />
studies in patients with multiple BCCs,<br />
especially individuals with BCNS.<br />
Readers interested in the trial can contact<br />
Maria Acosta, administrative coordinator<br />
at CHORI, for more information<br />
at macosta@chori.org.<br />
Photo courtesy of Robert H. Johr, MD<br />
and Women’s. 5 Women’s and Harvard Medical School.<br />
Nearly 113,000 participants<br />
STUDY PROVIDES NEW EVIDENCE FOR LINK BE-<br />
TWEEN CAFFEINE CONSUMPTION AND DECREASED<br />
RISK OF NON-MELANOMA SKIN CANCER<br />
An accumulating body of research<br />
demonstrates the health benefits of caffeine.<br />
In October 2011, a team from<br />
Brigham and Women’s Hospital and<br />
Harvard University showed that increased<br />
consumption of coffee led to<br />
a decrease in risk for non-melanoma<br />
skin cancer (NMSC). 5 A new study<br />
from another team at Brigham and<br />
Women’s further confirms the link between<br />
caffeine and a decreased risk of<br />
NMSC, particularly BCC. 6,7<br />
Lead researcher Jiali Han, PhD, an<br />
(112,897) were included in the present<br />
analysis; 22,786 developed BCC over<br />
the 20-plus years of study follow-up. 6<br />
<strong>The</strong> researchers observed an inverse association<br />
between all coffee consumption<br />
and the risk of BCC. 6 According<br />
to an abstract on the study in Cancer<br />
Research, “compared with individuals<br />
who consumed caffeinated coffee less<br />
than 1 cup per month, women who<br />
consumed more than 3 cups/d had<br />
the lowest risk (RR, 0.79; 95% CI,<br />
0.74–0.85; P trend<br />
< 0.0001) and the RR<br />
for men was 0.90 (95% CI, 0.80–1.01;<br />
P trend<br />
=0.003).” <strong>The</strong> abstract notes that<br />
caffeine from other sources, including<br />
tea, cola and chocolate, was also<br />
associate professor at Brigham and<br />
Women’s, Harvard Medical School and<br />
Harvard School of Public Health, and<br />
colleagues conducted a prospective<br />
analysis of data from the Nurses’ Health<br />
inversely associated with BCC risk. 7<br />
Decaffeinated coffee did not lead to a<br />
decreased risk of BCC. 7<br />
“Given the nearly 1 million new<br />
Study and the Health Professionals cases of BCC diagnosed each year in<br />
Follow-Up Study, both of which are<br />
large, long-running studies investigating<br />
the factors that influence women’s<br />
and men’s health, respectively. 6 <strong>The</strong><br />
Nurses’ Health Study was also used for<br />
the October 2011 study from Brigham<br />
the United States, daily dietary factors<br />
with even small protective effects<br />
may have great public health impact,”<br />
explains researcher Fengju Song, PhD,<br />
a postdoctoral fellow in the department<br />
of dermatology at Brigham and<br />
“Our study indicates that coffee consumption<br />
may be an important option<br />
to help prevent BCC.” n<br />
References<br />
1. National Cancer Institute. FDA approval for<br />
vismodegib. Available at: http://www.cancer.gov/<br />
cancertopics/druginfo/fda-vismodegib. Accessibility<br />
verified July 10, 2012.<br />
2. <strong>The</strong> <strong>Dermatologist</strong>. News and Trends: FDA approves<br />
Erivedge for advanced <strong>basal</strong> <strong>cell</strong> <strong>carcinoma</strong>. Available<br />
at: http://the-dermatologist.com/content/newsand-trends-2.<br />
Accessibility verified July 10, 2012.<br />
3. Sekulic A, Migden MR, Oro AE, et al. Efficacy<br />
and safety of vismodegib in advanced <strong>basal</strong> <strong>cell</strong> <strong>carcinoma</strong>.<br />
N Engl J Med. 2012;366(23):2171-2179.<br />
4. Tang JY, Mackay-Wiggan JM, Aszterbaum M,<br />
et al. Inhibiting the hedgehog pathway in patients<br />
with the <strong>basal</strong> <strong>cell</strong> nevus syndrome. N Engl J Med.<br />
2012;366(23):2180-2188.<br />
5. <strong>The</strong> <strong>Dermatologist</strong>. Additional evidence for the<br />
link between caffeine and decreased skin cancer<br />
risk. Available at: http://the-dermatologist.com/<br />
content/additional-evidence-link-between-caffeine-and-decreased-skin-cancer-risk.<br />
Accessibility<br />
verified July 10, 2012.<br />
6. American Association for Cancer Research. Coffee<br />
consumption inversely associated with risk of most<br />
common form of skin cancer. Available at: http://www.<br />
aacr.org/home/public--media/aacr-press-releases.<br />
aspx?d=2513. Accessibility verified July 10, 2012.<br />
7. Song F, Qureshi AA, Han J. Increased caffeine<br />
intake is associated with reduced risk of<br />
<strong>basal</strong> <strong>cell</strong> <strong>carcinoma</strong> of the skin. Cancer Res.<br />
2012;72(13):3282-3289.<br />
July 2012 | <strong>The</strong> <strong>Dermatologist</strong> ® | www.the-dermatologist.com E3