mass transfer in multiphase systems - Greenleaf University
mass transfer in multiphase systems - Greenleaf University
mass transfer in multiphase systems - Greenleaf University
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
MASS TRANSFER IN MULTIPHASE SYSTEMS: VOLATILE ORGANIC COMPOUND<br />
REMOVAL IN THREE-PHASE SYSTEMS<br />
35000<br />
30000<br />
System 1 Solid VOC Concentration vs Time<br />
Models<br />
X, ppb<br />
25000<br />
20000<br />
15000<br />
10000<br />
5000<br />
X data<br />
ln(X) vs t1/2<br />
X vs ln(t)<br />
Model ln(X) vs 1/t1/2<br />
Model ln(t)<br />
0<br />
0 20 40 60 80<br />
Time, hr<br />
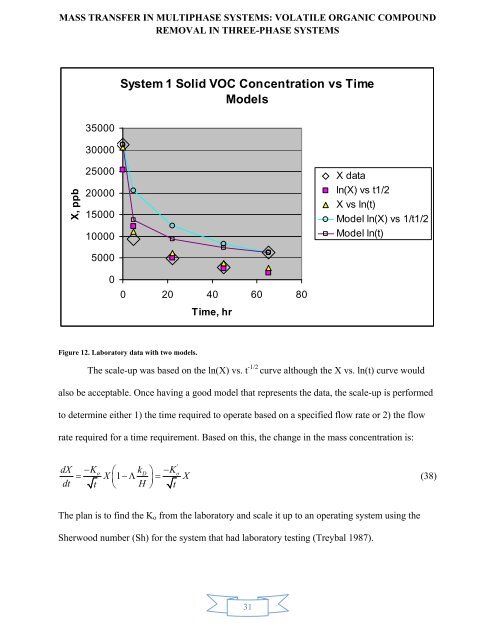
Figure 12. Laboratory data with two models.<br />
The scale-up was based on the ln(X) vs. t -1/2 curve although the X vs. ln(t) curve would<br />
also be acceptable. Once hav<strong>in</strong>g a good model that represents the data, the scale-up is performed<br />
to determ<strong>in</strong>e either 1) the time required to operate based on a specified flow rate or 2) the flow<br />
rate required for a time requirement. Based on this, the change <strong>in</strong> the <strong>mass</strong> concentration is:<br />
dX<br />
dt<br />
Ko<br />
kD<br />
K<br />
X 1 <br />
t H t<br />
'<br />
o<br />
X<br />
(38)<br />
The plan is to f<strong>in</strong>d the K o from the laboratory and scale it up to an operat<strong>in</strong>g system us<strong>in</strong>g the<br />
Sherwood number (Sh) for the system that had laboratory test<strong>in</strong>g (Treybal 1987).<br />
31