mass transfer in multiphase systems - Greenleaf University
mass transfer in multiphase systems - Greenleaf University
mass transfer in multiphase systems - Greenleaf University
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
MASS TRANSFER IN MULTIPHASE SYSTEMS: VOLATILE ORGANIC COMPOUND<br />
REMOVAL IN THREE-PHASE SYSTEMS<br />
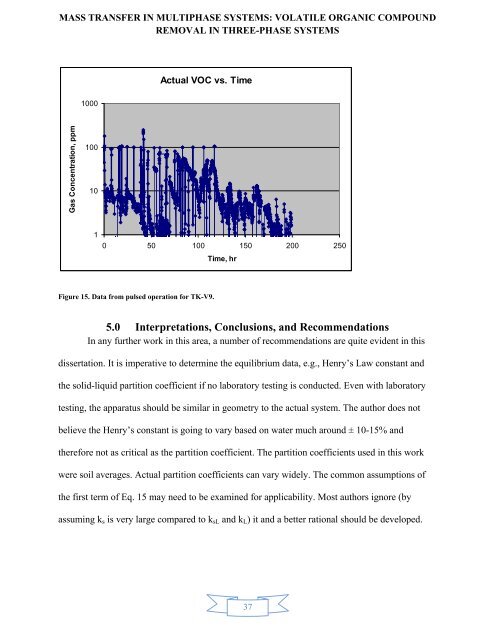
Actual VOC vs. Time<br />
1000<br />
Gas Concentration, ppm<br />
100<br />
10<br />
1<br />
0 50 100 150 200 250<br />
Time, hr<br />
Figure 15. Data from pulsed operation for TK-V9.<br />
5.0 Interpretations, Conclusions, and Recommendations<br />
In any further work <strong>in</strong> this area, a number of recommendations are quite evident <strong>in</strong> this<br />
dissertation. It is imperative to determ<strong>in</strong>e the equilibrium data, e.g., Henry’s Law constant and<br />
the solid-liquid partition coefficient if no laboratory test<strong>in</strong>g is conducted. Even with laboratory<br />
test<strong>in</strong>g, the apparatus should be similar <strong>in</strong> geometry to the actual system. The author does not<br />
believe the Henry’s constant is go<strong>in</strong>g to vary based on water much around ± 10-15% and<br />
therefore not as critical as the partition coefficient. The partition coefficients used <strong>in</strong> this work<br />
were soil averages. Actual partition coefficients can vary widely. The common assumptions of<br />
the first term of Eq. 15 may need to be exam<strong>in</strong>ed for applicability. Most authors ignore (by<br />
assum<strong>in</strong>g k s is very large compared to k sL and k L ) it and a better rational should be developed.<br />
37