Reference List - Gore Medical
Reference List - Gore Medical
Reference List - Gore Medical
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
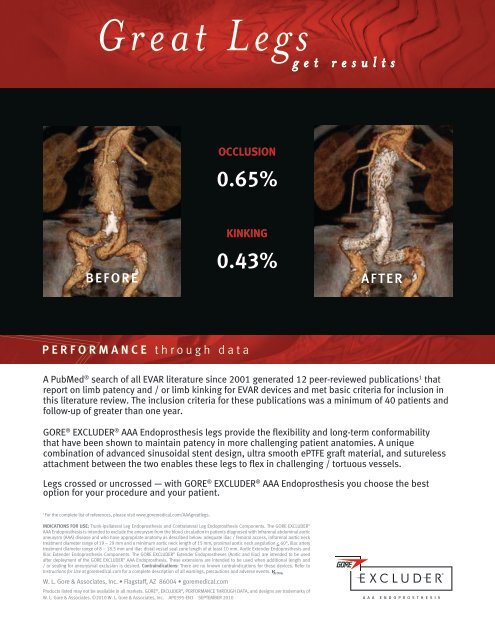
G r e a t L e g sg e t r e s u l t s<br />
OCCLUSION<br />
0.65%<br />
BEFORE<br />
KINKING<br />
0.43%<br />
AFTER<br />
P E R F O R M A N C E t h r o u g h d a t a<br />
A PubMed ® search of all EVAR literature since 2001 generated 12 peer-reviewed publications 1 that<br />
report on limb patency and / or limb kinking for EVAR devices and met basic criteria for inclusion in<br />
this literature review. The inclusion criteria for these publications was a minimum of 40 patients and<br />
follow-up of greater than one year.<br />
GORE ® EXCLUDER ® AAA Endoprosthesis legs provide the flexibility and long-term conformability<br />
that have been shown to maintain patency in more challenging patient anatomies. A unique<br />
combination of advanced sinusoidal stent design, ultra smooth ePTFE graft material, and sutureless<br />
attachment between the two enables these legs to flex in challenging / tortuous vessels.<br />
Legs crossed or uncrossed — with GORE ® EXCLUDER ® AAA Endoprosthesis you choose the best<br />
option for your procedure and your patient.<br />
1<br />
For the complete list of references, please visit www.goremedical.com/AAAgreatlegs.<br />
INDICATIONS FOR USE: Trunk-Ipsilateral Leg Endoprosthesis and Contralateral Leg Endoprosthesis Components. The GORE EXCLUDER ®<br />
AAA Endoprosthesis is intended to exclude the aneurysm from the blood circulation in patients diagnosed with infrarenal abdominal aortic<br />
aneurysm (AAA) disease and who have appropriate anatomy as described below: adequate iliac / femoral access, infrarenal aortic neck<br />
treatment diameter range of 19 – 29 mm and a minimum aortic neck length of 15 mm, proximal aortic neck angulation < 60°, iliac artery<br />
treatment diameter range of 8 – 18.5 mm and iliac distal vessel seal zone length of at least 10 mm. Aortic Extender Endoprosthesis and<br />
Iliac Extender Endoprosthesis Components. The GORE EXCLUDER ® Extender Endoprostheses (Aortic and Iliac) are intended to be used<br />
after deployment of the GORE EXCLUDER ® AAA Endoprosthesis. These extensions are intended to be used when additional length and<br />
/ or sealing for aneurysmal exclusion is desired. Contraindications: There are no known contraindications for these devices. Refer to<br />
Instructions for Use at goremedical.com for a complete description of all warnings, precautions and adverse events.<br />
W. L. <strong>Gore</strong> & Associates, Inc. • Flagstaff, AZ 86004 • goremedical.com<br />
Products listed may not be available in all markets. GORE ® , EXCLUDER ® , PERFORMANCE THROUGH DATA, and designs are trademarks of<br />
W. L. <strong>Gore</strong> & Associates. ©2010 W. L. <strong>Gore</strong> & Associates, Inc. AP0395-EN3 SEPTEMBER 2010
1. Bos WTGJ, Tielliu IFJ, van den Dungen JJAM, et al. Results of endovascular abdominal aortic aneurysm repair with selective use of the<br />
<strong>Gore</strong> Excluder. Journal of Cardiovascular Surgery 2009;50(2):159-164.<br />
2. Bos WT, Tielliu IF, Sondakh AO, Vourliotakis G, Bracale UM, Verhoeven EL. Hybrid endograft solution for complex iliac anatomy: zenith<br />
body and excluder limbs. Vascular 2010;18(3):136-140.<br />
3. Carroccio A, Faries PL, Morrissey NJ, et al. Predicting iliac limb occlusions after bifurcated aortic stent grafting: anatomic and devicerelated<br />
causes. Journal of Vascular Surgery 2002;36(4):679-684.<br />
4. Haider SE, Najjar SF, Cho J-S, et al. Sac behavior after aneurysm treatment with the <strong>Gore</strong> Excluder low-permeability aortic<br />
endoprosthesis: 12-month comparison to the original Excluder device. Journal of Vascular Surgery 2006;44(4):694-700.<br />
5. Hingorani AP, Ascher E, Marks N, et al. Iatrogenic injuries of the common femoral artery (CFA) and external iliac artery (EIA) during<br />
endograft placement: an underdiagnosed entity. Journal of Vascular Surgery 2009;50(3):505-509.<br />
6. Maleux G, Koolen M, Heye S, Nevelsteen A. Limb occlusion after endovascular repair of abdominal aortic aneurysms with supported<br />
endografts. Journal of Vascular & Interventional Radiology 2008;19(10):1409-1412.<br />
7. Maynar M, Zander T, Qian Z, et al. Bifurcated endoprosthesis for treatment of aortoiliac occlusive lesions. Journal of Endovascular<br />
Therapy 2005;12(1):22-27.<br />
8. Mendonça CT, de Carvalho CA, Weingärtner J, et al. Endovascular treatment of abdominal aortic aneurysms in high-surgical-risk patients<br />
using commercially available stent-grafts. Journal of Endovascular Therapy 2010;17(1):89-94.<br />
9. Peterson BG, Matsumura JS, Brewster DC, Makaroun MS. Five-year report of a multicenter controlled clinical trial of open versus<br />
endovascular treatment of abdominal aortic aneurysms. Journal of Vascular Surgery 2007;45(5):885-890.<br />
10. Pfammatter T, Lachat ML, Künzli A, et al. Short-term results of endovascular AAA repair with the Excluder Bifurcated Stent-Graft. Journal<br />
of Endovascular Therapy 2002;9(4):474-480.<br />
11. van Marrewijk CJ, Leurs LJ, Vallabhaneni SR, Harris PL, Buth J, Laheij RJF; EUROSTAR collaborators. Risk-adjusted outcome analysis of<br />
endovascular abdominal aortic aneurysm repair in a large population: how do stent-grafts compare? Journal of Endovascular Therapy<br />
2005;12(4):417-429.<br />
12. Verhoeven ELG, Tielliu FJ, Prins TR, et al. Frequency and outcome of re-interventions after endovascular repair for abdominal aortic<br />
aneurysm: a prospective cohort study. European Journal of Endovascular Surgery 2004;28(4):357-364.<br />
W. L. <strong>Gore</strong> & Associates, Inc.<br />
Flagstaff, AZ 86004<br />
+65.67332882 (Asia Pacific)<br />
00800.6334.4673 (Europe)<br />
800.437.8181 (United States)<br />
928.779.2771 (United States)<br />
goremedical.com<br />
Products listed may not be available in all markets.<br />
GORE, EXCLUDER ® , and designs are trademarks of W. L. GORE & Associates.<br />
© 2010 W. L. GORE & Associates, Inc. AP0618-EN1 SEPTEMBER 2010