A Great Fit For Anal Fistula Repair - Gore Medical
A Great Fit For Anal Fistula Repair - Gore Medical
A Great Fit For Anal Fistula Repair - Gore Medical
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
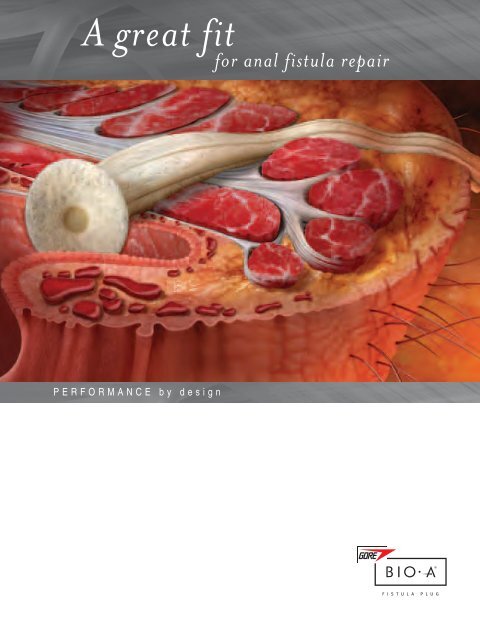
A great fit<br />
for anal fistula repair<br />
PERFORMANCE by design
We’ve met the challenge of<br />
anal fistula repair head-on.<br />
The management of anal fistula presents a challenge to the<br />
general and colorectal surgeon. The balance is finite between<br />
eradicating the anal fistula and maintaining anal continence.<br />
Traditional surgical techniques – such as fistulotomy,<br />
fistulectomy and other invasive procedures – divide and sever<br />
the sphincter, thereby increasing the risk of incontinence.<br />
The GORE ® BIO-A ® <strong>Fistula</strong> Plug provides a significant step<br />
forward in both technique and treatment. By providing a<br />
synthetic scaffold for soft tissue repair that facilitates tissue<br />
generation and healing, the GORE ® BIO-A ® <strong>Fistula</strong> Plug offers<br />
surgeons an alternative approach for sphincter-preserving anal<br />
fistula repair.<br />
Its patented design enables robust device placement, thereby<br />
reducing the likelihood of becoming dislodged. A single plug<br />
configuration is tailorable to fit most anal fistula shapes and<br />
sizes. Because it is engineered to conform to the anal fistula<br />
tract, the device reduces the potential for failure due to fall-out.<br />
GORE ® BIO-A ® <strong>Fistula</strong> Plug non-woven web<br />
creates a 3D porous structure with tunnels<br />
for cellular infiltration.
An Advanced, Easily Customized Design<br />
Robust device placement.<br />
The design of the GORE ® BIO-A ® <strong>Fistula</strong> Plug features<br />
bundled hollow tubes attached to a circular disk. The<br />
disk helps the plug stay in place, reducing the chance<br />
of the leading cause of anal fistula plug failure — the<br />
extrusion of the plug through the distal opening of<br />
the anal fistula tract. It also facilitates reproducible<br />
anchoring for dependable performance.<br />
Engineered to conform to the tract<br />
and reduce failure due to fall-out.<br />
The 3D structure of the tubes expands to fill the<br />
defect, snugly holding the device in place within the<br />
anal fistula.<br />
Minimizing risk of incontinence.<br />
<strong>Fistula</strong> repairs using the GORE ® BIO-A ® <strong>Fistula</strong><br />
Plug avoid dividing the sphincter muscles, thereby<br />
reducing the risk of incontinence. The fistula<br />
plug provides a scaffold for soft tissue repair and<br />
facilitates closure of the fistula.<br />
One configuration tailorable to fit<br />
most anal fistula shapes and sizes.<br />
The design of the GORE ® BIO-A ® <strong>Fistula</strong> Plug allows<br />
surgeons to configure the device for individual anal<br />
fistula geometries. Each tube can be trimmed or<br />
removed entirely, so the device conforms to most<br />
anal fistula shapes and sizes.
A Heritage of High Performance:<br />
A proven, synthetic 100% bioabsorbable material<br />
The GORE ® BIO-A ® <strong>Fistula</strong> Plug is a revolutionary combination of a proven, synthetic 100% bioabsorbable material 1 —<br />
Polyglycolic Acid:Trimethylene Carbonate (PGA:TMC): Without the risk associated with biologics, this synthetic tissue<br />
scaffold provides uniformity and consistency. This remarkably versatile polymer is chemically and mechanically stable<br />
and chemically inert.<br />
This 100% synthetic bioabsorbable scaffold facilitates tissue generation and healing. The PGA:TMC fibers form a 3D matrix<br />
of open, highly interconnected pores. Cells migrate into the scaffold and tissue is generated as the body gradually absorbs<br />
the material, leaving no permanent material in the body.<br />
Porous 3D matrix facilitates<br />
tissue generation and healing<br />
Porcine Submucosa<br />
Plug Surface (50x)<br />
GORE ® BIO-A ® <strong>Fistula</strong><br />
Plug Surface (50x)<br />
Material Replaced by Tissue at 1:1 Ratio 2<br />
Percent Volume of Defect<br />
100<br />
80<br />
60<br />
40<br />
20<br />
0<br />
Day of Implant 3 Months* 6 Months*<br />
GORE ® BIO-A ® Web<br />
Collagen<br />
*Cells & Blood Vessels Make Up Remaining Volume
More than 15 years of clinical experience<br />
GORE ® BIO-A ® Material is backed by 15 years of clinical use and <strong>Gore</strong> research, as well<br />
as clinical use in numerous parts of the body, including the mouth, abdominal wall,<br />
colon, stomach, heart, lung, liver, pancreas and spleen.<br />
GORE ® BIO-A ® <strong>Fistula</strong> Plug GORE ® SEAMGUARD ®<br />
Bioabsorbable Staple<br />
Line Reinforcement<br />
GORE ® BIO-A ® Tissue<br />
Reinforcement<br />
GORE ® BIO-A ® Hernia Plug
Frequently Asked Questions<br />
Specific questions regarding<br />
plug use:<br />
• Do you recommend debriding / curetting the tract?<br />
The standard of care prescribed by the surgeon should be<br />
used in this case. Gently debriding or curetting the tract creates<br />
a non-epithelialized wound bed which allows for the infiltration<br />
of cells that function in wound healing and regeneration of tissue.<br />
Care should be taken during debriding since it has the potential to<br />
enlarge or damage the anal fistula tract.<br />
• Do you close the internal opening of the anal fistula tract?<br />
Close the external opening(s)?<br />
The disk of the GORE ® BIO-A ® <strong>Fistula</strong> Plug is securely seated<br />
at the internal opening by being sutured to or covered with the<br />
patient’s rectal mucosa. To allow for natural drainage of the anal<br />
fistula tract in the post-operative period, the external (secondary)<br />
opening of the anal fistula tract should not be closed.<br />
• How can I tailor the device to fill the tract?<br />
Tubes can be trimmed off as needed to accommodate the<br />
diameter of the anal fistula tract. Six tubes can fill an anal fistula<br />
tract of approximately 6 mm in diameter.<br />
• What guidelines do you have on trimming the plug<br />
(disk or tubes)?<br />
It is not recommended to trim the disk. In trimming off tube(s)<br />
for sizing the plug for the anal fistula tract: remove the centermost<br />
tubes first. Take care to ensure that the disk lies flat and<br />
is well apposed to the rectal mucosa at the internal (primary)<br />
opening of the anal fistula tract.<br />
Questions regarding GORE ®<br />
BIO-A ® Material:<br />
What is GORE ® BIO-A ® Material?<br />
GORE ® BIO-A ® Material is a uniquely designed web of<br />
biocompatible polymers that is gradually absorbed by the<br />
body while its 3D matrix is replaced by vascularized soft tissue.<br />
As a synthetic bioabsorbable tissue scaffold, it is not derived<br />
from human or animal tissue but engineered for uniformity,<br />
consistency, and versatility in soft tissue reinforcement.<br />
• What is left once the material is absorbed?<br />
Cells infiltrate the 3D matrix and replace the web with<br />
vascularized soft tissue at an approximate 1:1 ratio as it absorbs<br />
over six months. Histology from animal 2 and human explants 3<br />
indicates the tissue is initially a mix of type I and type III collagen<br />
with maturation into primarily type I collagen, reflecting a normal<br />
wound healing process.<br />
• How is this different from a “biologic”?<br />
GORE ® BIO-A ® Material provides a non-permanent scaffold for<br />
tissue generation like biologics, but is made of bioabsorbable<br />
polymers (67% PGA:33% TMC). Due to its synthetic nature, the<br />
product is consistent and uniform with handling characteristics<br />
that facilitate placement and no risk of human or animal source<br />
contamination. It is easy to use with no operative preparation,<br />
such as soaking or stretching.<br />
• What can be expected post-operatively?<br />
It is common for patients to experience drainage during the<br />
post-operative healing period. Patients should be advised that<br />
they may experience discomfort during this period. Typically<br />
these symptoms will subside during the weeks following the<br />
procedure. The material of the plug is absorbed and tissue<br />
generation occurs over a six month period.<br />
Insist on a <strong>Great</strong> <strong>Fit</strong>.<br />
The GORE ® BIO-A ® <strong>Fistula</strong> Plug combines a proven, synthetic 100% bioabsorbable<br />
material 1 with a patented design engineered to conform to the tract.<br />
Insist on a great fit for anal fistula repair: the GORE ® BIO-A ® <strong>Fistula</strong> Plug.<br />
Product Name Size Catalogue Number<br />
GORE ® BIO-A ® <strong>Fistula</strong> Plug Six 9 cm tubes, 16 mm disk FP0616
W. L. <strong>Gore</strong> & Associates, Inc.<br />
Flagstaff, AZ 86004<br />
+65.67332882 (Asia Pacific)<br />
00800.6334.4673 (Europe)<br />
800.437.8181 (United States)<br />
928.779.2771 (United States)<br />
goremedical.com<br />
1<br />
Katz AR, Mukherjee DP, Kaganov AL, Gordon S. A new synthetic monofilament<br />
absorbable suture made from polytrimethylene carbonate. Surgery, Gynecology &<br />
Obstetrics 1985;161(3):213-222.<br />
2<br />
Morales-Conde S, Flores M, Fernández V, Morales-Méndez S. Bioabsorbable<br />
vs polypropylene plug for the “Mesh and Plug” inguinal hernia repair. Poster<br />
presented at the 9th Annual Meeting of the American Hernia<br />
Society; February 9-12, 2005; San Diego, CA.<br />
3<br />
Carl R. Doerhoff, MD, FACS, SurgiCare of Missouri, P.C., Jefferson City, Missouri,<br />
USA. Experience with the GORE ® BIO-A ® Hernia Plug in Inguinal Herniorrhaphy.<br />
Products listed may not be available in all markets. GORE ® , BIO-A ® ,<br />
PERFORMANCE BY DESIGN are trademarks of W. L. <strong>Gore</strong> & Associates.<br />
©2012 W. L. <strong>Gore</strong> & Associates, Inc. AQ2917-EN1 MAY 2012