Printable PDF Version - Gore Medical
Printable PDF Version - Gore Medical
Printable PDF Version - Gore Medical
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
This resulted in all three eyelets being<br />
successfully captured by the locking<br />
loop. There was no residual shunt<br />
around the device and its proper<br />
position was documented.<br />
The patient received 400 units / kg of<br />
heparin for 24 hours and 200 units / kg<br />
for another day. Antibiotics were<br />
repeated twice during the first 24 hours.<br />
Transthoracic echocardiography showed<br />
a good result with no residual shunt.<br />
The patient was dismissed 48 hours<br />
after the procedure.<br />
Discussion:<br />
This was our first implantation of<br />
<strong>Version</strong> 2.0 of the GORE ® HELEX Septal<br />
Occluder. Several modifications of<br />
the prior version have been made by<br />
W. L. <strong>Gore</strong> & Associates to improve<br />
the handling of the delivery system.<br />
Specifically, the mandrel has been<br />
reinforced by a stainless steel wire to<br />
prevent potential kinking. This issue<br />
intermittently occurred with the previous<br />
version when the distal end of the<br />
mandrel was sharply bent or pushed<br />
forward during the delivery procedure.<br />
The result of a kinked mandrel included<br />
an inability to properly configure the<br />
left-sided disc, thus alleviation of this<br />
potential issue with the new version of<br />
the device is a beneficial improvement.<br />
Despite the mandrel stiffener, it is still<br />
recommended to not push the mandrel<br />
forward during the procedure, which<br />
is under normal circumstances an<br />
unnecessary maneuver.<br />
As a second modification, the gray<br />
control catheter of the improved device<br />
now has a hydrophilic lubricious coating<br />
intended to decrease the frictional<br />
forces within the delivery catheter.<br />
This markedly improves the kinesthetic<br />
feel with which the device may be<br />
deployed. In our case, the decreased<br />
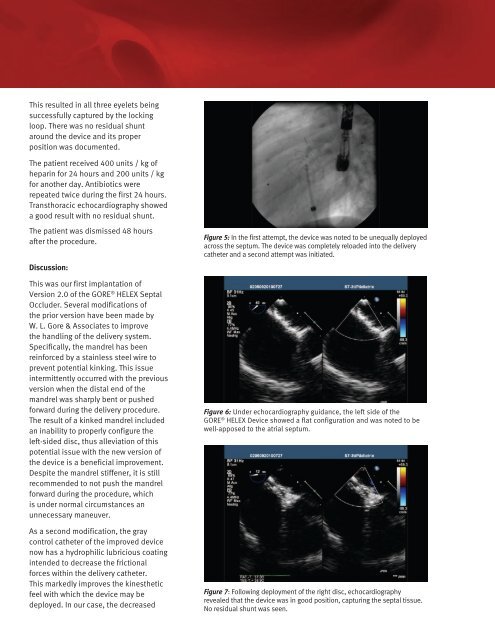
Figure 5: In the first attempt, the device was noted to be unequally deployed<br />
across the septum. The device was completely reloaded into the delivery<br />
catheter and a second attempt was initiated.<br />
Figure 6: Under echocardiography guidance, the left side of the<br />
GORE ® HELEX Device showed a flat configuration and was noted to be<br />
well-apposed to the atrial septum.<br />
Figure 7: Following deployment of the right disc, echocardiography<br />
revealed that the device was in good position, capturing the septal tissue.<br />
No residual shunt was seen.