Printable PDF Version - Gore Medical
Printable PDF Version - Gore Medical
Printable PDF Version - Gore Medical
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
U P C O M I N G E V E N T S<br />
Please Join Us...<br />
The Society for Cardiovascular Angiography (SCAI)<br />
May 4 – 7, 2011 Baltimore, Maryland<br />
Pediatric Inteventional Cardio Society (PICS)<br />
July 24 – 27, 2011 Boston, Massachusetts<br />
Congenital and Structural Interventions (CSI - iCi)<br />
June 22 – 25, 2011 Frankfurt, Germany<br />
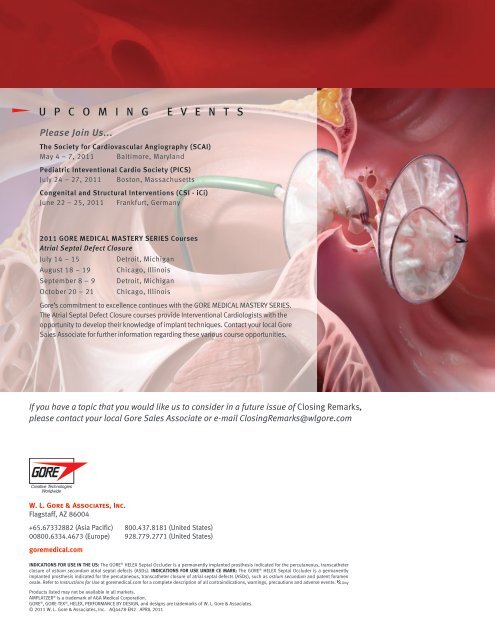
2011 GORE MEDICAL MASTERY SERIES Courses<br />
Atrial Septal Defect Closure<br />
July 14 – 15<br />
Detroit, Michigan<br />
August 18 – 19 Chicago, Illinois<br />
September 8 – 9 Detroit, Michigan<br />
October 20 – 21 Chicago, Illinois<br />
<strong>Gore</strong>’s commitment to excellence continues with the GORE MEDICAL MASTERY SERIES.<br />
The Atrial Septal Defect Closure courses provide Interventional Cardiologists with the<br />
opportunity to develop their knowledge of implant techniques. Contact your local <strong>Gore</strong><br />
Sales Associate for further information regarding these various course opportunities.<br />
If you have a topic that you would like us to consider in a future issue of Closing Remarks,<br />
please contact your local <strong>Gore</strong> Sales Associate or e-mail ClosingRemarks@wlgore.com<br />
W. L. <strong>Gore</strong> & Associates, Inc.<br />
Flagstaff, AZ 86004<br />
+65.67332882 (Asia Pacific) 800.437.8181 (United States)<br />
00800.6334.4673 (Europe) 928.779.2771 (United States)<br />
goremedical.com<br />
INDICATIONS FOR USE IN THE US: The GORE ® HELEX Septal Occluder is a permanently implanted prosthesis indicated for the percutaneous, transcatheter<br />
closure of ostium secundum atrial septal defects (ASDs). INDICATIONS FOR USE UNDER CE MARK: The GORE ® HELEX Septal Occluder is a permanently<br />
implanted prosthesis indicated for the percutaneous, transcatheter closure of atrial septal defects (ASDs), such as ostium secundum and patent foramen<br />
ovale. Refer to Instructions for Use at goremedical.com for a complete description of all contraindications, warnings, precautions and adverse events.<br />
Products listed may not be available in all markets.<br />
AMPLATZER ® is a trademark of AGA <strong>Medical</strong> Corporation.<br />
GORE ® , GORE-TEX ® , HELEX, PERFORMANCE BY DESIGN, and designs are trademarks of W. L. <strong>Gore</strong> & Associates.<br />
© 2011 W. L. <strong>Gore</strong> & Associates, Inc. AQ4478-EN2 APRIL 2011