Download Program and abstract book - 8th Conference on FFC ...
Download Program and abstract book - 8th Conference on FFC ...
Download Program and abstract book - 8th Conference on FFC ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Oral 24<br />
Abstract<br />
Using Relaxometry to Probe the Traditi<strong>on</strong>al SBM Paradigm<br />
Mark Woods <str<strong>on</strong>g>and</str<strong>on</strong>g> Mauro Botta<br />
Department of Chemistry, Portl<str<strong>on</strong>g>and</str<strong>on</strong>g> State University; Advanced Imaging Research Center,<br />
Oreg<strong>on</strong> Health <str<strong>on</strong>g>and</str<strong>on</strong>g> Science University; Dipartimento di Scienze e Innovazi<strong>on</strong>e Tecnologica,<br />
Università del Piem<strong>on</strong>te Orientale "A. Avogadro"<br />
It is now well established the relaxivity of Gd 3+ chelates is relatively well described<br />
by Solom<strong>on</strong> Bloembergen <str<strong>on</strong>g>and</str<strong>on</strong>g> Morgan theory. This in turn informs our underst<str<strong>on</strong>g>and</str<strong>on</strong>g>ing of the<br />
limitati<strong>on</strong>s <strong>on</strong> relaxivity of the low molecular<br />
weight Gd 3+ chelates used in clinical practice.<br />
These agents tumble rapidly in soluti<strong>on</strong> which<br />
limits their relaxivity. When this rotati<strong>on</strong>al<br />
restricti<strong>on</strong> is lifted by making the chelates tumble<br />
more slowly the relaxivity c<strong>on</strong>tinues to be limited<br />
by the slower than optimal water exchange<br />
kinetics of the chelate. Thus it has been widely<br />
understood that in order to maximize relaxivity it<br />
is necessary to both slow molecular tumbling <str<strong>on</strong>g>and</str<strong>on</strong>g><br />
optimize water exchange. This c<strong>on</strong>cept is often<br />
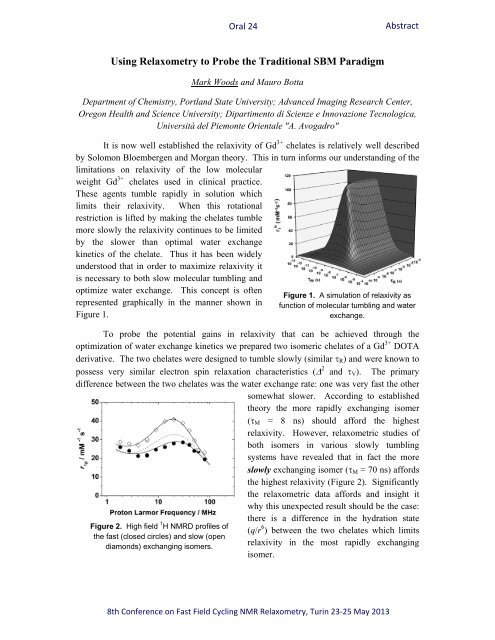
represented graphically in the manner shown in<br />
Figure 1.<br />
Figure 1. A simulati<strong>on</strong> of relaxivity as<br />
functi<strong>on</strong> of molecular tumbling <str<strong>on</strong>g>and</str<strong>on</strong>g> water<br />
exchange.<br />
To probe the potential gains in relaxivity that can be achieved through the<br />
optimizati<strong>on</strong> of water exchange kinetics we prepared two isomeric chelates of a Gd 3+ DOTA<br />
derivative. The two chelates were designed to tumble slowly (similar R ) <str<strong>on</strong>g>and</str<strong>on</strong>g> were known to<br />
possess very similar electr<strong>on</strong> spin relaxati<strong>on</strong> characteristics ( 2 <str<strong>on</strong>g>and</str<strong>on</strong>g> V ). The primary<br />
difference between the two chelates was the water exchange rate: <strong>on</strong>e was very fast the other<br />
somewhat slower. According to established<br />
theory the more rapidly exchanging isomer<br />
( M = 8 ns) should afford the highest<br />
relaxivity. However, relaxometric studies of<br />
both isomers in various slowly tumbling<br />
systems have revealed that in fact the more<br />
slowly exchanging isomer ( M = 70 ns) affords<br />
the highest relaxivity (Figure 2). Significantly<br />
the relaxometric data affords <str<strong>on</strong>g>and</str<strong>on</strong>g> insight it<br />
why this unexpected result should be the case:<br />
Figure 2. High field 1 H NMRD profiles of<br />
the fast (closed circles) <str<strong>on</strong>g>and</str<strong>on</strong>g> slow (open<br />
diam<strong>on</strong>ds) exchanging isomers.<br />
there is a difference in the hydrati<strong>on</strong> state<br />
(q/r 6 ) between the two chelates which limits<br />
relaxivity in the most rapidly exchanging<br />
isomer.<br />
<str<strong>on</strong>g>8th</str<strong>on</strong>g> <str<strong>on</strong>g>C<strong>on</strong>ference</str<strong>on</strong>g> <strong>on</strong> Fast Field Cycling NMR Relaxometry, Turin 23-25 May 2013