PROPERTIES OF CO2 AS A REFRIGERANT - Centro Studi Galileo
PROPERTIES OF CO2 AS A REFRIGERANT - Centro Studi Galileo
PROPERTIES OF CO2 AS A REFRIGERANT - Centro Studi Galileo
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
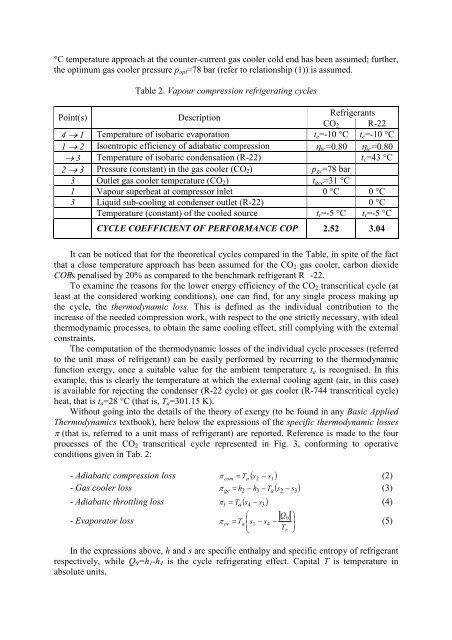
°C temperature approach at the counter-current gas cooler cold end has been assumed; further,<br />
the optimum gas cooler pressure p opt =78 bar (refer to relationship (1)) is assumed.<br />
Table 2. Vapour compression refrigerating cycles<br />
Point(s)<br />
Description<br />
Refrigerants<br />
CO 2 R-22<br />
4 1 Temperature of isobaric evaporation t e =-10 °C t e =-10 °C<br />
1 2 Isoentropic efficiency of adiabatic compression ic =0.80 ic =0.80<br />
3 Temperature of isobaric condensation (R-22) t c =43 °C<br />
2 3 Pressure (constant) in the gas cooler (CO 2 ) p gc =78 bar<br />
3 Outlet gas cooler temperature (CO 2 ) t gce =31 °C<br />
1 Vapour superheat at compressor inlet 0 °C 0 °C<br />
3 Liquid sub-cooling at condenser outlet (R-22) 0 °C<br />
Temperature (constant) of the cooled source t r =-5 °C t r =-5 °C<br />
CYCLE COEFFICIENT <strong>OF</strong> PERFORMANCE COP 2.52 3.04<br />
It can be noticed that for the theoretical cycles compared in the Table, in spite of the fact<br />
that a close temperature approach has been assumed for the CO 2 gas cooler, carbon dioxide<br />
COPis penalised by 20% as compared to the benchmark refrigerant R -22.<br />
To examine the reasons for the lower energy efficiency of the CO 2 transcritical cycle (at<br />
least at the considered working conditions), one can find, for any single process making up<br />
the cycle, the thermodynamic loss. This is defined as the individual contribution to the<br />
increase of the needed compression work, with respect to the one strictly necessary, with ideal<br />
thermodynamic processes, to obtain the same cooling effect, still complying with the external<br />
constraints.<br />
The computation of the thermodynamic losses of the individual cycle processes (referred<br />
to the unit mass of refrigerant) can be easily performed by recurring to the thermodynamic<br />
function exergy, once a suitable value for the ambient temperature t a is recognised. In this<br />
example, this is clearly the temperature at which the external cooling agent (air, in this case)<br />
is available for rejecting the condenser (R-22 cycle) or gas cooler (R-744 transcritical cycle)<br />
heat, that is t a =28 °C (that is, T a =301.15 K).<br />
Without going into the details of the theory of exergy (to be found in any Basic Applied<br />
Thermodynamics textbook), here below the expressions of the specific thermodynamic losses<br />
(that is, referred to a unit mass of refrigerant) are reported. Reference is made to the four<br />
processes of the CO 2 transcritical cycle represented in Fig. 3, conforming to operative<br />
conditions given in Tab. 2:<br />
- Adiabatic compression loss com = T a ( s 2 s 1 )<br />
(2)<br />
- Gas cooler loss gc = h2 h3<br />
Ta<br />
( s2<br />
s3<br />
)<br />
(3)<br />
- Adiabatic throttling loss = ( )<br />
(4)<br />
- Evaporator loss<br />
t T a s 4 s 3<br />
Q0<br />
<br />
<br />
<br />
ev = Ta<br />
s <br />
1 s4<br />
(5)<br />
Tr<br />
<br />
In the expressions above, h and s are specific enthalpy and specific entropy of refrigerant<br />
respectively, while Q 0 =h 1 -h 4 is the cycle refrigerating effect. Capital T is temperature in<br />
absolute units.