RNA interference: traveling in the cell and gaining functions?
RNA interference: traveling in the cell and gaining functions?
RNA interference: traveling in the cell and gaining functions?
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
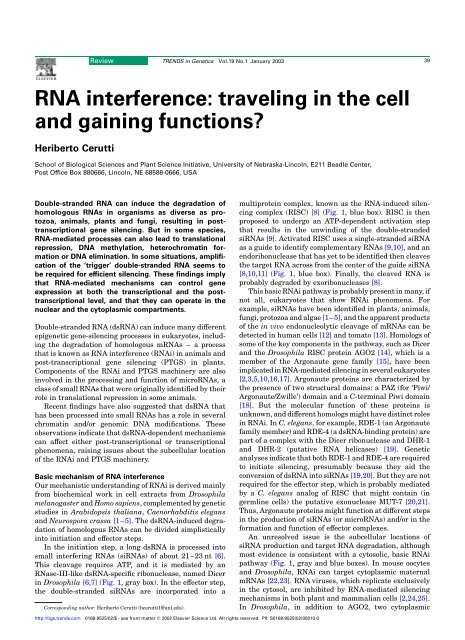
40Review TRENDS <strong>in</strong> Genetics Vol.19 No.1 January 2003ss<strong>RNA</strong> anneal<strong>in</strong>gto antisense <strong>RNA</strong>?DNAMethylationInvertedrepeattransgeneSensetransgene?Transposableelement'aberrant'ss<strong>RNA</strong>?Primer<strong>in</strong>dependentRdRP?<strong>RNA</strong> syn<strong>the</strong>sis??Repressivechromat<strong>in</strong>structureShortor longds<strong>RNA</strong>?PPPPds<strong>RNA</strong> CAF? si<strong>RNA</strong> RISC-like?ActivatedRISC-like??NucleusCytoplasm?P<strong>RNA</strong> degradationExogenousds<strong>RNA</strong>Viralds<strong>RNA</strong>DicerPPPPds<strong>RNA</strong> si<strong>RNA</strong> RISCActivatedPrimerRISCdependentRdRP?An A nPP<strong>RNA</strong> syn<strong>the</strong>sis<strong>RNA</strong> degradationTRENDS <strong>in</strong> GeneticsFig. 1. Models of molecular pathways <strong>in</strong>volved <strong>in</strong> double-str<strong>and</strong>ed <strong>RNA</strong> (ds<strong>RNA</strong>)-mediated silenc<strong>in</strong>g. The basic mechanism, which is probably present <strong>in</strong> most eukaryotesundergo<strong>in</strong>g ds<strong>RNA</strong>-mediated silenc<strong>in</strong>g, is <strong>in</strong>dicated by <strong>the</strong> gray <strong>and</strong> blue boxes. The rema<strong>in</strong><strong>in</strong>g steps seem to occur <strong>in</strong> at least some organisms, but <strong>the</strong>ir generality is currentlyunknown <strong>and</strong> <strong>the</strong>ir sub<strong>cell</strong>ular location is ma<strong>in</strong>ly hypo<strong>the</strong>tical. The ‘triggers’ of silenc<strong>in</strong>g, ei<strong>the</strong>r direct sources of ds<strong>RNA</strong> or transcription units produc<strong>in</strong>g s<strong>in</strong>glestr<strong>and</strong>ed<strong>RNA</strong>s that can be presumably converted to ds<strong>RNA</strong>, are colored red. Green <strong>RNA</strong>, endogenously transcribed s<strong>in</strong>gle-str<strong>and</strong>ed <strong>RNA</strong>; purple <strong>RNA</strong>, <strong>RNA</strong> syn<strong>the</strong>sized bya putative <strong>RNA</strong>-directed <strong>RNA</strong> polymerase; blue <strong>and</strong> red <strong>RNA</strong>, double-str<strong>and</strong>ed <strong>RNA</strong> <strong>in</strong>troduced exogenously or result<strong>in</strong>g from viral replication, anneal<strong>in</strong>g of complementaryss<strong>RNA</strong>s <strong>and</strong>/or hairp<strong>in</strong> <strong>RNA</strong>. Prote<strong>in</strong>s or prote<strong>in</strong> complexes are <strong>in</strong>dicated by yellow boxes: CAF, an Arabidopsis homolog of Dicer; Dicer, an RNase-III-like ds<strong>RNA</strong>-specificribonuclease; RdRP, an <strong>RNA</strong>-directed <strong>RNA</strong> polymerase; <strong>and</strong> RISC, <strong>RNA</strong>-<strong>in</strong>duced silenc<strong>in</strong>g complex. Although ds<strong>RNA</strong> is depicted <strong>in</strong> s<strong>in</strong>gle nuclear <strong>and</strong> cytoplasmic pools,depend<strong>in</strong>g on <strong>the</strong> source <strong>the</strong>se molecules might be delivered differently to <strong>the</strong> process<strong>in</strong>g Dicer enzymes. Similarly, <strong>the</strong> RISC <strong>and</strong> RISC-like complexes might have differentcomponents <strong>and</strong> associated effector prote<strong>in</strong>s depend<strong>in</strong>g on <strong>the</strong>ir <strong>functions</strong>. Although a role for ds<strong>RNA</strong> <strong>in</strong> direct<strong>in</strong>g methylation of homologous DNA sequences has beendemonstrated <strong>in</strong> plants, <strong>the</strong> molecular mach<strong>in</strong>ery <strong>in</strong>volved <strong>in</strong> this process <strong>and</strong> <strong>the</strong> actual nature of <strong>the</strong> ‘guide’ <strong>RNA</strong> have not been resolved. Recent evidence suggests that<strong>the</strong> RISC complex is equivalent to <strong>the</strong> miRNP complex (Fig. 2) <strong>in</strong> human <strong>cell</strong>s [17].Argonaute family members, AGO1 <strong>and</strong> Auberg<strong>in</strong>e (Aub),have been implicated <strong>in</strong> <strong>RNA</strong>i [26–28] (Table 1). In human<strong>cell</strong>s, <strong>RNA</strong>i seems to occur predom<strong>in</strong>antly <strong>in</strong> <strong>the</strong> cytosol[10,17], because a nucleoplasm conf<strong>in</strong>ed transcript iscompletely resistant to degradation <strong>in</strong>duced by a homologoussi<strong>RNA</strong> [29]. In addition, <strong>the</strong> only known human homolog ofDicer is located <strong>in</strong> <strong>the</strong> cytoplasm of HeLa <strong>cell</strong>s [30] (Table 1).Notably, <strong>the</strong> rat homolog of eIF2C2 (GERp95), an Argonauteprote<strong>in</strong> <strong>and</strong> a component of human RISC [10,17],isaperipheral membrane prote<strong>in</strong> that is associated primarilywith <strong>the</strong> Golgi complex or <strong>the</strong> endoplasmic reticulum [31].Exogenous (<strong>in</strong>jected or fed) ds<strong>RNA</strong> <strong>and</strong> viral ds<strong>RNA</strong>probably enter <strong>the</strong> cytoplasmic <strong>RNA</strong>i pathway directly(Fig. 1). In several organisms, however, <strong>RNA</strong>i can also be<strong>in</strong>duced by <strong>in</strong>verted repeat transgenes that are transcribed<strong>in</strong>to hairp<strong>in</strong> ds<strong>RNA</strong> <strong>in</strong> <strong>the</strong> nucleus [1,2,16]. Thisds<strong>RNA</strong>probably needs to be exported to <strong>the</strong> cytosol to targethomologous m<strong>RNA</strong>s effectively (Fig. 1). In both plants [32]<strong>and</strong> Drosophila [33], for example, <strong>in</strong>verted repeat transgenessilence more efficiently when <strong>the</strong> hairp<strong>in</strong> ds<strong>RNA</strong>conta<strong>in</strong>s an <strong>in</strong>tron <strong>and</strong> polyadenylation signals thatpresumably facilitate entry of <strong>the</strong> ds<strong>RNA</strong> <strong>in</strong>to <strong>the</strong> m<strong>RNA</strong>export pathway [34]. By contrast, a transgene designed toproduce non-polyadenylated, <strong>in</strong>tronless hairp<strong>in</strong> ds<strong>RNA</strong> –which is presumably reta<strong>in</strong>ed <strong>in</strong> nuclei – targets <strong>the</strong>degradation of a homologous m<strong>RNA</strong> very poorly [1,35].Potential amplification processes <strong>in</strong> <strong>RNA</strong>i <strong>and</strong> PTGSGenetic analyses suggest that an amplification step mightbe required for efficient <strong>RNA</strong>-mediated silenc<strong>in</strong>g <strong>in</strong> severalhttp://tigs.trends.com
Review TRENDS <strong>in</strong> Genetics Vol.19 No.1 January 2003 41Table 1. Genetic <strong>and</strong>/or biochemical components of <strong>the</strong> <strong>RNA</strong>i/PTGS mach<strong>in</strong>ery discussed <strong>in</strong> <strong>the</strong> text a,bGene function A. thaliana C. elegans C. re<strong>in</strong>hardtii D. discoideum D. melanogaster H. sapiens N. crassa S. pombeRNase III þ <strong>RNA</strong> helicase CAF DCR-1 Dicer Dicer [C] DCR1Unknown (Argonaute) AGO1 RDE-1,AGO1 [C], eIF2C1, QDE2 AGO1ALG-1,ALG-2AGO2,AUB [C],PIWI [N]eIF2C2Putative RdRP SDE1/SGS2 RRF-1,RRPA QDE1 RDP1 [N]RRF-3<strong>RNA</strong> helicaseDRH-1,DRH2MUT6 [N]Gem<strong>in</strong>3[N þ C]ds<strong>RNA</strong>-b<strong>in</strong>d<strong>in</strong>g prote<strong>in</strong>RDE-4RNase D likeMUT-7RecQ DNA helicaseQDE3UnknownGem<strong>in</strong>4[N þ C]a This list is not comprehensive <strong>and</strong> only some prote<strong>in</strong>s are <strong>in</strong>dicated for each of <strong>the</strong> follow<strong>in</strong>g organisms: Arabidopsis thaliana, Caenorhabditis elegans, Chlamydomonasre<strong>in</strong>hardtii, Dictyostelium discoideum, Drosophila melanogaster, Homo sapiens, Neurospora crassa, Schizosaccharomyces pombe.b Where known, <strong>the</strong> sub<strong>cell</strong>ular localization of <strong>the</strong> prote<strong>in</strong>s is shown <strong>in</strong> paren<strong>the</strong>ses: C, cytoplasmic; N, nuclear.systems. Recent studies <strong>in</strong> C. elegans have led to a model <strong>in</strong>which primary si<strong>RNA</strong>s (derived from <strong>the</strong> trigger ds<strong>RNA</strong>)might prime <strong>the</strong> syn<strong>the</strong>sis of additional ds<strong>RNA</strong>, us<strong>in</strong>g <strong>the</strong>target m<strong>RNA</strong> as a template, <strong>in</strong> a reaction catalyzed by aputative <strong>RNA</strong>-directed <strong>RNA</strong> polymerase (RdRP) [4,36](Fig. 1, cytoplasm). The newly syn<strong>the</strong>sized ds<strong>RNA</strong> would<strong>the</strong>n be cleaved by Dicer to generate secondary si<strong>RNA</strong>s at asufficient concentration to achieve efficient target m<strong>RNA</strong>degradation by RISC [4,36]. In support of this model, <strong>the</strong><strong>in</strong>jection of short antisense <strong>RNA</strong> oligomers <strong>in</strong>to C. eleganscan trigger silenc<strong>in</strong>g of endogenous genes, <strong>and</strong> this effect isdependent on a functional Dicer (DCR-1) [37].Putative homologs of a tomato RdRP are required forPTGS triggered by sense transgenes <strong>in</strong> Arabidopsis, forquell<strong>in</strong>g <strong>in</strong> Neurospora, <strong>and</strong> for <strong>RNA</strong>i <strong>in</strong> C. elegans <strong>and</strong>Dictyostelium discoideum [1–4,36,38–40]; however, biochemicalactivity rema<strong>in</strong>s to be demonstrated def<strong>in</strong>itivelyfor any of <strong>the</strong> RdRP homologs implicated <strong>in</strong> <strong>RNA</strong>i <strong>and</strong>PTGS [5]. In addition, <strong>in</strong> both Drosophila <strong>and</strong> humans, <strong>the</strong>results of many experiments argue aga<strong>in</strong>st an obligatoryrole for an RdRP <strong>in</strong> ds<strong>RNA</strong>-<strong>in</strong>duced <strong>RNA</strong>i [5,9,12],although an RdRP-like activity has been reported <strong>in</strong>extracts from Drosophila embryos [41].Because si<strong>RNA</strong> production <strong>and</strong> target m<strong>RNA</strong> cleavagemost probably occur <strong>in</strong> <strong>the</strong> cytosol, it is tempt<strong>in</strong>g tospeculate that <strong>the</strong> RdRP-dependent amplification stepdescribed <strong>in</strong> C. elegans also occurs <strong>in</strong> <strong>the</strong> cytoplasm (Fig. 1).Notably, RDE-1 <strong>and</strong> RDE-4 are required to <strong>in</strong>itiate <strong>RNA</strong>i<strong>in</strong>duced by <strong>in</strong>jected or transgene-produced long ds<strong>RNA</strong>s[20], but not for silenc<strong>in</strong>g triggered by short antisense<strong>RNA</strong>s [37]. Thus, <strong>the</strong> RdRP-generated ds<strong>RNA</strong> might bedelivered to Dicer differently to <strong>the</strong> way <strong>in</strong> whichexogenous or transgenic ds<strong>RNA</strong> is delivered [19] (Fig. 1,cytoplasm). In Dictyostelium, RRPA, a putative RdRPrequired for <strong>RNA</strong>i, conta<strong>in</strong>s an N-term<strong>in</strong>al <strong>RNA</strong> helicasedoma<strong>in</strong> that has not been found <strong>in</strong> o<strong>the</strong>r RdRPs [40].Intrigu<strong>in</strong>gly, <strong>the</strong> closest homolog of this helicase doma<strong>in</strong> isthat of C. elegans Dicer, suggest<strong>in</strong>g that doma<strong>in</strong> swapp<strong>in</strong>gmight have occurred between Dictyostelium Dicer <strong>and</strong>RRPA [40]. If this is true, <strong>the</strong>n perhaps Dicer <strong>and</strong> an RdRP<strong>in</strong>teract <strong>in</strong> a complex such that <strong>the</strong> ds<strong>RNA</strong> generated byRdRP activity is immediately accessible <strong>and</strong> rapidlyprocessed by Dicer.In C. elegans, RDE-1, RDE-4 <strong>and</strong> RRF-1 (a putativeRdRP) are required for ds<strong>RNA</strong>-<strong>in</strong>duced silenc<strong>in</strong>g <strong>in</strong> <strong>the</strong>soma, even when <strong>the</strong> trigger ds<strong>RNA</strong> is expressed from atransgene <strong>in</strong> <strong>the</strong> nuclei of target tissues [16,19,36]. SDE1/SGS2 (a putative RdRP) <strong>and</strong> AGO1 (<strong>the</strong> first describedmember of <strong>the</strong> Argonaute family [15]) are also requiredfor PTGS <strong>in</strong>duced by sense transgenes <strong>in</strong> Arabidopsis[38,39,42]. They are, however, completely dispensable forPTGS <strong>in</strong>duced by <strong>the</strong> expression of <strong>in</strong>verted repeat transgenesthat produce hairp<strong>in</strong> ds<strong>RNA</strong> [42]. This suggests that<strong>the</strong> putative amplification step catalyzed by ArabidopsisSDE1/SGS2 might be different to that mediated byC. elegans RRF-1 [42].In plants, <strong>the</strong>re is also an SDE1/SGS2-dependentspread<strong>in</strong>g of silenc<strong>in</strong>g from <strong>the</strong> region homologous to <strong>the</strong>trigger ds<strong>RNA</strong> <strong>in</strong>to <strong>the</strong> adjacent non-homologous 5 0 <strong>and</strong> 3 0regions of a target transgene [43]. This is not consistentwith <strong>the</strong> simple notion that primary si<strong>RNA</strong>s prime 5 0 ! 3 0ds<strong>RNA</strong> syn<strong>the</strong>sis on sense m<strong>RNA</strong>. A possible explanationis that trigger ds<strong>RNA</strong>s or si<strong>RNA</strong>s <strong>in</strong>teract with <strong>the</strong>transgene DNA <strong>and</strong> <strong>in</strong>duce changes <strong>in</strong> chromat<strong>in</strong> structure[38,43] (Fig. 1, nucleus, repressive chromat<strong>in</strong> structure).This would lead to <strong>the</strong> production of ‘aberrant’, butnearly full-length transgenic <strong>RNA</strong>s that could be used bySDE1/SGS2 for primer-<strong>in</strong>dependent amplification [38,43](Fig. 1, nucleus, ‘aberrant ss<strong>RNA</strong>?’).In several plant species, PTGS of transgenes usuallycorrelates with DNA methylation <strong>in</strong> transcribed regions[1–3,44]. Chromat<strong>in</strong> modifications associated with (orpreced<strong>in</strong>g) DNA methylation might lead to <strong>the</strong> productionof truncated aberrant <strong>RNA</strong>s, as has been reported <strong>in</strong>filamentous fungi [45]. A role for DNA methylation <strong>and</strong>/orchromat<strong>in</strong> modification <strong>in</strong> PTGS of sense transgenes isalso supported by <strong>the</strong> observation that mutations <strong>in</strong>MET1/DDM2 (a DNA methyltransferase) <strong>and</strong> <strong>in</strong> DDM1(a member of <strong>the</strong> SWI2/SNF2 family of chromat<strong>in</strong>remodel<strong>in</strong>gprote<strong>in</strong>s) can affect both <strong>the</strong> degree <strong>and</strong>persistence of post-transcriptional silenc<strong>in</strong>g <strong>in</strong> Arabidopsis[44], although <strong>in</strong>direct effects, such as saturation of <strong>the</strong>PTGS mach<strong>in</strong>ery by <strong>the</strong> unregulated expression ofendogenous <strong>RNA</strong>s, cannot be ruled out.The nature of <strong>the</strong> postulated aberrant <strong>RNA</strong> rema<strong>in</strong>sunknown [1,2,5,38], but PTGS can be <strong>in</strong>duced <strong>in</strong> soybeanhttp://tigs.trends.com
42Review TRENDS <strong>in</strong> Genetics Vol.19 No.1 January 2003<strong>and</strong> tobacco by transgenes express<strong>in</strong>g ribozyme-truncatedtranscripts that are not polyadenylated <strong>and</strong> are preferentiallyreta<strong>in</strong>ed <strong>in</strong> <strong>the</strong> nucleus [46].InDrosophila, <strong>the</strong>post-transcriptional silenc<strong>in</strong>g of alcohol dehydrogenasetransgenes – a phenomenon that is similar to PTGS<strong>in</strong>duced by sense transgenes <strong>in</strong> plants – is dependent on<strong>the</strong> Argonaute family member Piwi [47]. Notably, Piwi islocalized predom<strong>in</strong>antly <strong>in</strong> <strong>the</strong> nucleus of <strong>in</strong>terphase <strong>cell</strong>s[48] (Table 1). In addition, <strong>in</strong> Schizosaccharomyces pombe,RDP1 (a putative RdRP homolog) is associated withcentromeric DNA [49] (Table 1).It is <strong>the</strong>refore tempt<strong>in</strong>g to speculate that <strong>in</strong> Arabidopsis<strong>the</strong> mechanism dependent on SDE1/SGS2 <strong>and</strong> AGO1might function to produce ds<strong>RNA</strong> from non-polyadenylated,prematurely term<strong>in</strong>ated or misprocessed <strong>RNA</strong>s <strong>in</strong><strong>the</strong> nuclear compartment (Fig. 1, nucleus, ‘primer <strong>in</strong>dependentRdRP?’). Perhaps <strong>the</strong> RdRP itself operates as a sensorfor <strong>the</strong> accumulation of aberrant <strong>RNA</strong>s above a certa<strong>in</strong>threshold [5]. The result<strong>in</strong>g ds<strong>RNA</strong> could be ei<strong>the</strong>r processedto si<strong>RNA</strong>s by a nuclear Dicer (see below) or exported to <strong>the</strong>cytoplasm as part of a ribonucleoprote<strong>in</strong> complex thatdelivers <strong>the</strong> ds<strong>RNA</strong> to a cytosolic Dicer. But only <strong>the</strong> lattermight be able to <strong>in</strong>duce PTGS because, as discussed above,ds<strong>RNA</strong> from <strong>in</strong>verted repeat transgenes apparently needsto be exported for efficient silenc<strong>in</strong>g [32,33].A similar model could expla<strong>in</strong> co-suppression triggeredby s<strong>in</strong>gle-copy dispersed transgenes as well as certa<strong>in</strong>transposon <strong>and</strong> repetitive DNA-silenc<strong>in</strong>g phenomena[3,4,13,19,38]. But it should be noted that some aberrant<strong>RNA</strong>s could exit <strong>the</strong> nucleus <strong>and</strong> <strong>the</strong> SDE1/SGS2-dependent step could occur <strong>in</strong> <strong>the</strong> cytosol. Fur<strong>the</strong>rmore,this RdRP might have dual roles <strong>in</strong> both <strong>the</strong> nuclear <strong>and</strong><strong>the</strong> cytoplasmic compartments.The apparent differences <strong>in</strong> <strong>the</strong> requirement or <strong>the</strong>characteristics of an amplification step, when compar<strong>in</strong>gArabidopsis, C. elegans, Drosophila <strong>and</strong> human, mightreflect true mechanistic differences among organisms.Alternatively, at least <strong>in</strong> species <strong>in</strong> which RdRPs homologsare encoded by multigene families, such as Arabidopsis,Dictyostelium, Neurospora <strong>and</strong> C. elegans [3,4,36,38–40],different family members might participate <strong>in</strong> differentsteps of <strong>the</strong> pathway. Thus, <strong>the</strong> discrepancies mightultimately reflect our current lack of underst<strong>and</strong><strong>in</strong>g of<strong>the</strong> role of several components. In addition, as mentionedabove, <strong>the</strong> enzymatic activity of <strong>the</strong> putative RdRPs<strong>in</strong>volved <strong>in</strong> <strong>RNA</strong>i <strong>and</strong> PTGS rema<strong>in</strong>s to be verified. Thisis particularly relevant because loss of function of RRF-3(a possible RdRP) <strong>in</strong> C. elegans enhances <strong>the</strong> sensitivityto <strong>RNA</strong>i <strong>in</strong> several tissues [50]. Thus, RRF-3 behaves asa negative modulator of <strong>the</strong> <strong>RNA</strong>i response, which isdifficult to reconcile with its postulated activity (but seeRef. [50]).Nuclear phenomena associated with <strong>RNA</strong>i <strong>and</strong> PTGSIn C. elegans, <strong>RNA</strong>i mediated by <strong>in</strong>jected long ds<strong>RNA</strong>virtually abolished <strong>the</strong> accumulation of a homologouscytoplasmic transcript, but it also produced a substantialreduction of nascent <strong>RNA</strong> <strong>in</strong> <strong>the</strong> nucleus [51]. <strong>RNA</strong>i is alsotriggered by ds<strong>RNA</strong> homologous to some <strong>in</strong>trons, suggest<strong>in</strong>gthat at least certa<strong>in</strong> precursor m<strong>RNA</strong>s (prem<strong>RNA</strong>s)can be targeted for degradation [52].http://tigs.trends.comIn <strong>the</strong> green alga Chlamydomonas re<strong>in</strong>hardtii, MUT6(a DEAH-box <strong>RNA</strong> helicase required for PTGS [53]) islocalized <strong>in</strong> <strong>the</strong> nucleus (K. van Dijk, B. Jeong <strong>and</strong>H. Cerutti, unpublished; Table 1). This helicase associateswith a s<strong>in</strong>gle-str<strong>and</strong>ed <strong>RNA</strong>-b<strong>in</strong>d<strong>in</strong>g prote<strong>in</strong> <strong>and</strong> with aribonuclease conta<strong>in</strong><strong>in</strong>g a staphylococcal nuclease-likedoma<strong>in</strong>. Because MUT6 is required for <strong>the</strong> degradationof non-polyadenylated transposon transcripts [53], whichare presumably reta<strong>in</strong>ed <strong>in</strong> nuclei, <strong>the</strong>se observationssuggest <strong>the</strong> existence of a nuclear RISC-like complex(Fig. 1, nucleus). In organisms with a s<strong>in</strong>gle, cytoplasmicDicer, RISC or a similar ribonucleoprote<strong>in</strong> (RNP) complexmight be imported <strong>in</strong>to <strong>the</strong> nucleus. But evidence fromC. elegans suggests that most pre-m<strong>RNA</strong>s do not seem tobe targeted by <strong>RNA</strong>i [51,52], possibly because <strong>the</strong>y areprotected by RNP complexes <strong>and</strong>/or rapidly processed tomature m<strong>RNA</strong>s <strong>and</strong> exported to <strong>the</strong> cytosol [34]. Thus, anuclear RISC-like complex might be capable of degrad<strong>in</strong>gonly certa<strong>in</strong> transcripts, such as non-polyadenylatedtransposon <strong>RNA</strong>s or accessible pre-m<strong>RNA</strong>s.In human <strong>cell</strong>s, <strong>RNA</strong>i seems to be limited mostly to <strong>the</strong>cytoplasm [10,17,29]. A nucleoplasm-sequestered transcriptwas found to be resistant to degradation <strong>in</strong>duced bya homologous si<strong>RNA</strong>. But <strong>the</strong> same <strong>RNA</strong>, <strong>in</strong> <strong>the</strong> biochemicallydef<strong>in</strong>ed nuclear fraction, was partiallydegraded when undergo<strong>in</strong>g export from <strong>the</strong> nucleus [29].A possible explanation is that transcripts become accessibleto <strong>the</strong> cytoplasmic <strong>RNA</strong>i mach<strong>in</strong>ery before completetransit of <strong>the</strong> m<strong>RNA</strong>s through <strong>the</strong> nuclear pores [29].Alternatively, nuclear RISC-like complexes might exist <strong>in</strong>human <strong>cell</strong>s but only target accessible transcripts, such asthose that undergo remodel<strong>in</strong>g of associated prote<strong>in</strong>sconcomitant with nuclear export [34]. As suggestedpreviously [54], a fraction of <strong>the</strong> RISC complexes mightbe located at <strong>the</strong> nuclear pores <strong>and</strong> act as gatekeepers,scann<strong>in</strong>g <strong>the</strong> <strong>RNA</strong>s as <strong>the</strong>y are be<strong>in</strong>g exported.The human RISC complex is proposed to conta<strong>in</strong>eIF2C1 <strong>and</strong>/or eIF2C2, Gem<strong>in</strong>3 (a putative DEAD-box<strong>RNA</strong> helicase) <strong>and</strong> Gem<strong>in</strong>4 [10,17]. Gem<strong>in</strong>3 <strong>and</strong> Gem<strong>in</strong>4are also components of ano<strong>the</strong>r multiprote<strong>in</strong> complex,conta<strong>in</strong><strong>in</strong>g <strong>the</strong> SMN (survival of motor neurons) prote<strong>in</strong>,that <strong>functions</strong> <strong>in</strong> <strong>the</strong> assembly <strong>and</strong> restructur<strong>in</strong>g of diverseRNP particles <strong>in</strong>volved <strong>in</strong> transcription, m<strong>RNA</strong> splic<strong>in</strong>g<strong>and</strong> r<strong>RNA</strong> process<strong>in</strong>g [55,56]. Gem<strong>in</strong>3 <strong>and</strong> Gem<strong>in</strong>4 arelocalized both <strong>in</strong> <strong>the</strong> nucleus <strong>and</strong> <strong>in</strong> <strong>the</strong> cytosol [56],whereas <strong>the</strong> rat homolog of eIF2C2 is associated ma<strong>in</strong>lywith Golgi or endoplasmic reticulum membranes [31](Table 1). The sub<strong>cell</strong>ular location of <strong>the</strong> whole RISCcomplex rema<strong>in</strong>s to be determ<strong>in</strong>ed.The Arabidopsis genome encodes four putative Dicerhomologs [57,58]. One of <strong>the</strong>se, Carpel Factory (CAF), hasbeen implicated recently <strong>in</strong> <strong>the</strong> generation of micro<strong>RNA</strong>s[59], <strong>and</strong> it is predicted to be a nuclear prote<strong>in</strong> [60]. Thus,at least <strong>in</strong> Arabidopsis, ds<strong>RNA</strong> might also be processed tosi<strong>RNA</strong>s <strong>in</strong> <strong>the</strong> nucleus (Fig. 1). Indeed, non-polyadenylatedtransgenic hairp<strong>in</strong> ds<strong>RNA</strong>, which is designed to<strong>in</strong>hibit <strong>the</strong> transcription of homologous promoters <strong>and</strong> ispresumably reta<strong>in</strong>ed <strong>in</strong> <strong>the</strong> nucleus, is processed to small<strong>RNA</strong>s [1,35]. Putative si<strong>RNA</strong>s correspond<strong>in</strong>g to a viroid,which is replicated <strong>in</strong> <strong>the</strong> nucleus by <strong>the</strong> host <strong>RNA</strong>polymerase II, have also been detected <strong>in</strong> tomato [61]. In
Review TRENDS <strong>in</strong> Genetics Vol.19 No.1 January 2003 43addition, HC-Pro, a cytoplasmic viral suppressor of PTGS,<strong>in</strong>hibits <strong>the</strong> production of si<strong>RNA</strong>s associated with <strong>the</strong> posttranscriptionalsilenc<strong>in</strong>g of transgenes [2,62] but does notreduce <strong>the</strong> small <strong>RNA</strong>s correspond<strong>in</strong>g to promoterdirectedds<strong>RNA</strong> <strong>in</strong> tobacco [1,35].Recent work <strong>in</strong>volv<strong>in</strong>g viral suppressors of <strong>RNA</strong>mediatedsilenc<strong>in</strong>g <strong>in</strong> Nicotiana benthamiana <strong>and</strong>silenc<strong>in</strong>g-defective Arabidopsis mutants has demonstrated<strong>the</strong> existence of two size classes of si<strong>RNA</strong>s [58].Long (24–26 nt) si<strong>RNA</strong>s are correlated with systemicsilenc<strong>in</strong>g <strong>in</strong> N. benthamiana <strong>and</strong> methylation of retroelementsequences <strong>in</strong> Arabidopsis [58]. Retrotransposonsi<strong>RNA</strong>s <strong>in</strong> both plant species correspond exclusively to thislong class. By contrast, short (21–22 nt) si<strong>RNA</strong>s arecorrelated with sequence-specific degradation of a transgenicm<strong>RNA</strong> [58]. All of <strong>the</strong>se observations support <strong>the</strong>existence of at least two populations of small <strong>RNA</strong>s <strong>in</strong>plants (<strong>in</strong> addition to micro<strong>RNA</strong>s). Because subtle alterations<strong>in</strong> Dicer structure have been postulated to alter <strong>the</strong>spac<strong>in</strong>g between <strong>the</strong> catalytic centers [3], long <strong>and</strong> shortsi<strong>RNA</strong>s might be processed by different Dicer homologs,which are presumably located <strong>in</strong> <strong>the</strong> nuclear (for longsi<strong>RNA</strong>s?) or <strong>in</strong> <strong>the</strong> cytoplasmic (for short si<strong>RNA</strong>s?)compartments.In several plant species, ds<strong>RNA</strong> can direct methylationof homologous DNA sequences [1,2,35]. Methylation ofgenomic DNA occurs even when silenc<strong>in</strong>g is <strong>in</strong>duced by<strong>RNA</strong> viruses, with sequences homologous to nuclear DNA,that replicate exclusively <strong>in</strong> <strong>the</strong> cytoplasm [1,2,54]. Thisaga<strong>in</strong> suggests that <strong>the</strong>re is communication between <strong>the</strong>cytoplasm <strong>and</strong> <strong>the</strong> nucleus. When <strong>the</strong> ds<strong>RNA</strong> hashomology to a promoter, it <strong>in</strong>duces transcriptional silenc<strong>in</strong>g<strong>in</strong> association with DNA methylation [1,35]; however,it is not known whe<strong>the</strong>r long ds<strong>RNA</strong> or processed small<strong>RNA</strong>s are <strong>in</strong>volved <strong>in</strong> this process (Fig. 1, nucleus).Connections between <strong>the</strong> <strong>RNA</strong>i <strong>and</strong> <strong>the</strong> PTGS mach<strong>in</strong>ery<strong>and</strong> chromat<strong>in</strong> <strong>and</strong>/or genomic DNA modifications arealso start<strong>in</strong>g to emerge <strong>in</strong> o<strong>the</strong>r organisms. In C. elegans,mutations <strong>in</strong> <strong>the</strong> putative <strong>RNA</strong> exonuclease MUT-7reactivate transgenic arrays that are silenced by apolycomb-dependent, presumably transcriptional, mechanism[16]. Some polycomb group homologs, which arenormally <strong>in</strong>volved <strong>in</strong> chromat<strong>in</strong> repression, are alsorequired for <strong>RNA</strong>i under certa<strong>in</strong> experimental conditions[63]. Likewise, QDE3, a putative RecQ DNA helicase, isnecessary for quell<strong>in</strong>g <strong>in</strong> Neurospora [64]. InDrosophila,mutations <strong>in</strong> Piwi block PTGS <strong>and</strong> one aspect oftranscriptional silenc<strong>in</strong>g [47].Several recent reports have directly implicated <strong>the</strong><strong>RNA</strong>i <strong>and</strong> PTGS mach<strong>in</strong>ery <strong>in</strong> heterochromat<strong>in</strong> formation<strong>and</strong> genome rearrangements [49,65–68] (Fig. 1, nucleus).In many eukaryotes, heterochromat<strong>in</strong> is characterizedby a high density of histone H3 methylated at lys<strong>in</strong>e 9(H3-Lys9) [69]. This modification results <strong>in</strong> <strong>the</strong> b<strong>in</strong>d<strong>in</strong>g tohistone H3 of heterochromat<strong>in</strong> prote<strong>in</strong> 1 (HP1), <strong>and</strong>presumably o<strong>the</strong>r factors, <strong>and</strong> formation of a transcriptionallyrepressive chromat<strong>in</strong> structure [69]. H3-Lys9 methylationalso leads to DNA methylation <strong>in</strong> Neurospora <strong>and</strong>Arabidopsis [69]. But DNA methylation might be a secondarymodification that contributes, <strong>in</strong> certa<strong>in</strong> organisms, to<strong>the</strong> stability <strong>and</strong> <strong>in</strong>heritability of <strong>the</strong> silent chromat<strong>in</strong>state.In S. pombe, deletion of DCR1 (a Dicer homolog), RDP1(a putative RdRP homolog) or AGO1 (an Argonautehomolog) results <strong>in</strong> <strong>the</strong> transcriptional derepression oftransgenes <strong>in</strong>tegrated <strong>in</strong> centromeric regions, <strong>the</strong> loss ofH3-Lys9 methylation <strong>and</strong> <strong>the</strong> dissociation of SWI6 (<strong>the</strong>S. pombe homolog of HP1) from this chromat<strong>in</strong> doma<strong>in</strong>[49]. In addition, small <strong>RNA</strong>s complementary to bothstr<strong>and</strong>s of <strong>the</strong> centromeric repeats are detected <strong>in</strong> wildtype<strong>cell</strong>s [65]. These observations have led to a model <strong>in</strong>which ds<strong>RNA</strong> orig<strong>in</strong>at<strong>in</strong>g from <strong>the</strong> pericentric repeats bybi-directional transcription is processed to si<strong>RNA</strong>s, which<strong>in</strong> turn <strong>in</strong>duce H3-Lys9 methylation <strong>and</strong> heterochromat<strong>in</strong>formation [49]. Analysis of repetitive DNA <strong>in</strong>tegrated <strong>in</strong>toa euchromatic region has shown that <strong>the</strong> <strong>RNA</strong>i mach<strong>in</strong>eryis required for <strong>in</strong>itiat<strong>in</strong>g but not ma<strong>in</strong>ta<strong>in</strong><strong>in</strong>g <strong>the</strong> heterochromaticstate [66].Studies <strong>in</strong> <strong>the</strong> ciliated protozoan Tetrahymena <strong>the</strong>rmophilasupport <strong>the</strong> model of heterochromat<strong>in</strong> formationpostulated for S. pombe [67,68]. Tetrahymena conta<strong>in</strong>s atranscriptionally <strong>in</strong>active micronucleus (with an <strong>in</strong>tactgenome), which gives rise to a transcriptionally activemacronucleus with extensive deletions correspond<strong>in</strong>g toabout 15% of <strong>the</strong> micronuclear genome. This programmedDNA rearrangement is impaired <strong>in</strong> a mutant with defects<strong>in</strong> a homolog of Piwi [67]. Wild-type but not mutant <strong>cell</strong>sconta<strong>in</strong> small <strong>RNA</strong>s that hybridize preferentially tomicronucleus-specific sequences [67]. In addition, H3-Lys9methylation is required for <strong>the</strong> targeted DNA elim<strong>in</strong>ation<strong>in</strong> Tetrahymena [68]. It is thought that <strong>the</strong> small<strong>RNA</strong>s, which are processed from bi-directionally transcribedmicronucleus-specific sequences, scan <strong>the</strong> macronucleargenome, <strong>the</strong>reby direct<strong>in</strong>g H3-Lys9 methylation<strong>and</strong> subsequent genomic deletions [67,68]. Although small<strong>RNA</strong>s presumably target chromat<strong>in</strong> modification througha pair<strong>in</strong>g mechanism, <strong>the</strong> recognition step <strong>and</strong> <strong>the</strong>components that l<strong>in</strong>k small <strong>RNA</strong>s to histone modificationare currently unclear. Never<strong>the</strong>less, <strong>the</strong>se observationsextend <strong>the</strong> range of ds<strong>RNA</strong>-mediated processes <strong>and</strong>encourage <strong>the</strong> exam<strong>in</strong>ation of transcriptional regulationby ds<strong>RNA</strong>.Micro<strong>RNA</strong>sMicro<strong>RNA</strong>s constitute a class of noncod<strong>in</strong>g small <strong>RNA</strong>sthat are phylogenetically widespread <strong>in</strong> <strong>in</strong>vertebrates,vertebrates <strong>and</strong> plants [55,57,59,70–72]. The smalltemporal <strong>RNA</strong>s (st<strong>RNA</strong>s) l<strong>in</strong>-4 <strong>and</strong> let-7, which belong toa subclass of mi<strong>RNA</strong>s, were <strong>in</strong>itially identified <strong>in</strong> C. elegansas essential regulators of <strong>the</strong> tim<strong>in</strong>g of development [70].Mature st<strong>RNA</strong>s are about 21 nt <strong>and</strong> can form imperfectduplexes with sequences <strong>in</strong> <strong>the</strong> 3 0 untranslated regions oftarget m<strong>RNA</strong>s, lead<strong>in</strong>g to translational repression [70].Although <strong>the</strong> biological role of most mi<strong>RNA</strong>s is unknown,<strong>the</strong>y were thought orig<strong>in</strong>ally to function <strong>in</strong> translationalcontrol or o<strong>the</strong>r undef<strong>in</strong>ed mechanisms of genetic regulationthat are clearly dist<strong>in</strong>ct from <strong>the</strong> <strong>RNA</strong> cleavagedirected by si<strong>RNA</strong>s. But several new f<strong>in</strong>d<strong>in</strong>gs are blurr<strong>in</strong>g<strong>the</strong> differences between mi<strong>RNA</strong>s <strong>and</strong> si<strong>RNA</strong>s [17,71,72].Micro<strong>RNA</strong>s are usually recovered as s<strong>in</strong>gle-str<strong>and</strong>ed<strong>RNA</strong>s of 20–25 nt that have been processed from longerhttp://tigs.trends.com
44Review TRENDS <strong>in</strong> Genetics Vol.19 No.1 January 2003stem-loop precursors. In animals, precursor mi<strong>RNA</strong>s (premi<strong>RNA</strong>s)are apparently encoded as imperfectly pair<strong>in</strong>g<strong>in</strong>verted repeats of 60–70 nt [55,70]. In plants, however,some predicted pre-mi<strong>RNA</strong>s are more than three timeslonger than those <strong>in</strong> animals <strong>and</strong> <strong>in</strong>volve more extensive orcomplex stem-loop structures [57,59]. Most pre-mi<strong>RNA</strong>sare encoded <strong>in</strong> <strong>in</strong>tergenic regions <strong>and</strong> are probablytranscribed from autonomous promoters [57,59,70](Fig. 2); however, several pre-mi<strong>RNA</strong>s are clustered <strong>in</strong>genomic regions <strong>and</strong> are apparently syn<strong>the</strong>sized as as<strong>in</strong>gle polycistronic <strong>RNA</strong> [70,73]. In addition, a fewmi<strong>RNA</strong>s might also be processed from <strong>in</strong>trons, asbyproducts of pre-m<strong>RNA</strong> splic<strong>in</strong>g, or even from putativeprote<strong>in</strong>-cod<strong>in</strong>g <strong>RNA</strong>s [57,59,70].In mammalian <strong>cell</strong>s, maturation of mi<strong>RNA</strong>s <strong>in</strong>volves atleast two steps [73]. Both s<strong>in</strong>gle <strong>and</strong> clustered mi<strong>RNA</strong>sseem to be expressed as longer transcripts that areprocessed <strong>in</strong>to pre-mi<strong>RNA</strong>s of 60–70 nt <strong>in</strong> <strong>the</strong> nucleus[73] (Fig. 2, nucleus, ‘pre-process<strong>in</strong>g’). The pre-mi<strong>RNA</strong>sare <strong>the</strong>n exported to <strong>the</strong> cytoplasm <strong>and</strong> processed by Dicer,<strong>and</strong> possibly by o<strong>the</strong>r factors, <strong>in</strong>to mature mi<strong>RNA</strong>s ofabout 21 nt [73] (Fig. 2, cytoplasm). Recently, numeroushuman mi<strong>RNA</strong>s have been found to be associated <strong>in</strong> a 15SRNP complex that <strong>in</strong>cludes Gem<strong>in</strong>3, Gem<strong>in</strong>4 <strong>and</strong> eIF2C2[55]. The human homolog of let-7 is a component of thisMonocistronicunitTRENDS <strong>in</strong> GeneticsPre-process<strong>in</strong>gCAFPre-mi<strong>RNA</strong>RNP?Pre-mi<strong>RNA</strong>PolycistronicunitDicerPmi<strong>RNA</strong>NucleusCytoplasmPmi<strong>RNA</strong>A nP<strong>RNA</strong>degradationIntron conta<strong>in</strong>edunitFunction(s)?PmiRNP?miRNP PA nPTranslationrepressionFig. 2. Model of <strong>the</strong> orig<strong>in</strong> <strong>and</strong> potential <strong>functions</strong> of micro<strong>RNA</strong>s. The <strong>in</strong>dicatedpathways are ma<strong>in</strong>ly supported by experimental evidence <strong>in</strong> Arabidopsis, Caenorhabditiselegans, Drosophila <strong>and</strong> human. Green <strong>RNA</strong>, endogenously transcribeds<strong>in</strong>gle-str<strong>and</strong>ed <strong>RNA</strong>; red <strong>RNA</strong>, micro<strong>RNA</strong>s <strong>and</strong> micro<strong>RNA</strong> precursors. Prote<strong>in</strong> orprote<strong>in</strong> complexes are <strong>in</strong>dicated by yellow boxes: miRNP, ribonucleoprote<strong>in</strong> complexconta<strong>in</strong><strong>in</strong>g human Gem<strong>in</strong>3, Gem<strong>in</strong>4 <strong>and</strong> eIF2C2. Several steps related tomi<strong>RNA</strong> biogenesis <strong>and</strong> function are hypo<strong>the</strong>tical (see text for details). Recent evidencesuggests that <strong>the</strong> miRNP complex is equivalent to <strong>the</strong> RISC complex (Fig. 1)<strong>in</strong> human <strong>cell</strong>s [17].?microRNP (miRNP) <strong>and</strong> can direct <strong>the</strong> cleavage of aperfectly complementary target <strong>RNA</strong> [17]. These observationshave led to <strong>the</strong> proposal that <strong>the</strong> miRNP is <strong>the</strong>human RISC <strong>and</strong> can carry out both target cleavage <strong>in</strong> <strong>the</strong><strong>RNA</strong>i pathway <strong>and</strong> translational control <strong>in</strong> <strong>the</strong> mi<strong>RNA</strong>pathway [17].The biogenesis of at least some plant mi<strong>RNA</strong>s seems tobe different to that <strong>in</strong> animals. Although CAF, a homolog ofDicer, has been implicated <strong>in</strong> <strong>the</strong> generation of mi<strong>RNA</strong>s <strong>in</strong>Arabidopsis [59], pre-mi<strong>RNA</strong>s are detected poorly or not atall <strong>in</strong> Arabidopsis, whereas mature mi<strong>RNA</strong>s are observedreadily [57,59]. In addition, whereas <strong>in</strong> metazoans premi<strong>RNA</strong>s<strong>in</strong>crease when Dicer activity is reduced [74],mutants of CAF show significantly lower levels of mi<strong>RNA</strong>swithout any concomitant accumulation of precursor [59].This suggests that because CAF is predicted to be anuclear prote<strong>in</strong> [60], at least some plant mi<strong>RNA</strong>s might beprocessed ei<strong>the</strong>r co-transcriptionally or shortly aftertranscription from transient primary transcripts (Fig. 2,nucleus). Mature mi<strong>RNA</strong>s might <strong>the</strong>n be transported to<strong>the</strong> cytosol as part of a RNP complex <strong>and</strong>/or <strong>the</strong>y mighthave a role <strong>in</strong> <strong>the</strong> nuclear compartment (Fig. 2).Unlike animal mi<strong>RNA</strong>s, whose targets are difficult toidentify by sequence complementarity, some Arabidopsis<strong>and</strong> rice mi<strong>RNA</strong>s have been found to show perfect ornear-perfect complementarity to potential m<strong>RNA</strong> targets[71,72]. This suggested that <strong>the</strong>y might function as si<strong>RNA</strong>s[71,72]. Indeed, an Arabidopsis mi<strong>RNA</strong> (miR171/mi<strong>RNA</strong>39)was found to direct specific cleavage of complementarytranscripts correspond<strong>in</strong>g to a family of transcriptionalregulators [72]. Thus, despite <strong>the</strong> presence of mi<strong>RNA</strong>s <strong>in</strong>both plants <strong>and</strong> animals, current studies imply differences<strong>in</strong> <strong>the</strong> structure of predicted precursor <strong>RNA</strong>s, <strong>in</strong> <strong>the</strong> tim<strong>in</strong>g<strong>and</strong> (presumed) sub<strong>cell</strong>ular localization of process<strong>in</strong>g <strong>and</strong>,possibly, <strong>in</strong> <strong>the</strong> actual function of mature mi<strong>RNA</strong>s.Biological <strong>functions</strong> of <strong>RNA</strong>-mediated silenc<strong>in</strong>gMutational <strong>in</strong>activation of components of <strong>the</strong> <strong>RNA</strong>i <strong>and</strong>PTGS mach<strong>in</strong>ery affects at least three dist<strong>in</strong>ct eukaryoticprocesses: <strong>the</strong> defense response aga<strong>in</strong>st viruses, transposonmobility <strong>and</strong> <strong>the</strong> development of multi<strong>cell</strong>ular organisms[1–5,54]. The <strong>RNA</strong>i <strong>and</strong> PTGS processes wereorig<strong>in</strong>ally proposed to have evolved to counteract genomicparasites [1–4,54,75], but it is becom<strong>in</strong>g apparent thatds<strong>RNA</strong>-mediated mechanisms are also <strong>in</strong>volved <strong>in</strong> <strong>the</strong>normal regulation of endogenous genes. Drosophila malesuse an <strong>RNA</strong>i mechanism to degrade Stellate transcripts[75]; one mi<strong>RNA</strong> has been implicated <strong>in</strong> <strong>the</strong> specificcleavage of target m<strong>RNA</strong>s correspond<strong>in</strong>g to <strong>the</strong> SCARE-CROW-like transcription factors <strong>in</strong> Arabidopsis [72]; <strong>and</strong>several mi<strong>RNA</strong>s that are complementary to prote<strong>in</strong>-cod<strong>in</strong>gsequences have been identified <strong>in</strong> Arabidopsis <strong>and</strong> rice[57,59,71].Intrigu<strong>in</strong>gly, a high proportion of <strong>the</strong> predicted mi<strong>RNA</strong>targets function as developmental regulators <strong>in</strong> plants,suggest<strong>in</strong>g that mi<strong>RNA</strong>s might have a role <strong>in</strong> coord<strong>in</strong>at<strong>in</strong>ggrowth <strong>and</strong> development [71]. Consistent with this<strong>in</strong>terpretation, Arabidopsis CAF mutants, which aredefective <strong>in</strong> mi<strong>RNA</strong> process<strong>in</strong>g, show pronounced developmentalalterations [59,60].InC. elegans, developmentaldefects result<strong>in</strong>g from reduced function of Dicer <strong>and</strong> <strong>the</strong>http://tigs.trends.com
Review TRENDS <strong>in</strong> Genetics Vol.19 No.1 January 2003 45Argonaute-like prote<strong>in</strong>s ALG-1 <strong>and</strong> ALG-2 have also beenattributed to <strong>the</strong> improper process<strong>in</strong>g of mi<strong>RNA</strong> precursors<strong>and</strong> a reduction <strong>in</strong> mature st<strong>RNA</strong> expression [3,74].Itis <strong>the</strong>refore tempt<strong>in</strong>g to propose <strong>the</strong> existence of ancient,mi<strong>RNA</strong>-mediated mechanisms that regulate endogenousgenes <strong>in</strong> eukaryotes. But most endogenous small <strong>RNA</strong>scloned from animals, <strong>and</strong> several from plants, do notmatch prote<strong>in</strong>-cod<strong>in</strong>g or structural <strong>RNA</strong>s <strong>and</strong> <strong>the</strong>irmechanistic roles, with <strong>the</strong> exception of C. elegans st<strong>RNA</strong>s,rema<strong>in</strong> unknown [55,70,74].Dicer processes precursor ds<strong>RNA</strong>s to make both si<strong>RNA</strong>s<strong>and</strong> mi<strong>RNA</strong>s. In organisms encod<strong>in</strong>g only one Dicer, as<strong>in</strong>gle pathway might h<strong>and</strong>le small <strong>RNA</strong>s. In o<strong>the</strong>r words,<strong>the</strong> mi<strong>RNA</strong> <strong>and</strong> si<strong>RNA</strong> pathways might be <strong>in</strong>terchangeablefrom biogenesis of <strong>the</strong> small <strong>RNA</strong> to <strong>in</strong>teraction withits target. The f<strong>in</strong>al outcome, such as m<strong>RNA</strong> degradationor, for example, translational repression, would depend on<strong>the</strong> degree of complementarity to <strong>the</strong> target <strong>RNA</strong> <strong>and</strong>,presumably, on associated effector prote<strong>in</strong>s [3,17]. Thismodel is consistent with <strong>the</strong> f<strong>in</strong>d<strong>in</strong>gs that short hairp<strong>in</strong><strong>RNA</strong>s, resembl<strong>in</strong>g mi<strong>RNA</strong> precursors, can <strong>in</strong>duce <strong>RNA</strong>i onperfectly homologous target m<strong>RNA</strong>s [3,17], <strong>and</strong> that <strong>the</strong>human RISC seems to be equivalent to <strong>the</strong> 15S miRNPthat is associated with many mi<strong>RNA</strong>s [17,55]. Alternatively,two dist<strong>in</strong>ct pathways, <strong>in</strong>tersect<strong>in</strong>g at <strong>the</strong> Dicercatalyzed step, might be <strong>in</strong>volved <strong>in</strong> <strong>the</strong> generation <strong>and</strong>function of at least some mi<strong>RNA</strong>s <strong>and</strong> si<strong>RNA</strong>s [3]. This issupported by <strong>the</strong> requirement of different Argonauteprote<strong>in</strong>s for <strong>the</strong> production of functional st<strong>RNA</strong>s or si<strong>RNA</strong>s<strong>in</strong> C. elegans [16,19,74]. In organisms where Dicer isencoded by a multigene family, such as Arabidopsis [57,58],cytoplasmic <strong>and</strong> nuclear process<strong>in</strong>g pathways mightoperate.Recent f<strong>in</strong>d<strong>in</strong>gs have also implicated small <strong>RNA</strong>s <strong>in</strong>chromat<strong>in</strong> <strong>and</strong>/or DNA modifications <strong>and</strong> genomerearrangements, such as heterochromat<strong>in</strong> formation<strong>in</strong> S. pombe <strong>and</strong> DNA elim<strong>in</strong>ation <strong>in</strong> Tetrahymena[1,35,49,65–68]. This suggests that ds<strong>RNA</strong>-mediatedprocesses might have a role <strong>in</strong> genome organization <strong>and</strong>transcriptional control. It is clear that despite muchprogress result<strong>in</strong>g from a comb<strong>in</strong>ation of genetics <strong>and</strong>biochemistry, we are only just beg<strong>in</strong>n<strong>in</strong>g to underst<strong>and</strong> <strong>the</strong>mechanistic complexity of <strong>RNA</strong>-mediated silenc<strong>in</strong>g, itsbiological implications, <strong>and</strong> <strong>the</strong> differences <strong>and</strong> similaritiesamong different eukaryotes.AcknowledgementsI thank S. Jacobsen, T. Clemente <strong>and</strong> members of mylaboratory for helpful comments. My apologies to colleagueswhose work could not be cited due to spacelimitations. Supported by NIH grant GM62915.References1 Matzke, M. et al. (2001) <strong>RNA</strong>: guid<strong>in</strong>g gene silenc<strong>in</strong>g. Science 293,1080–10832 Vance, V. <strong>and</strong> Vaucheret, H. (2001) <strong>RNA</strong> silenc<strong>in</strong>g <strong>in</strong> plants – defense<strong>and</strong> counterdefense. Science 292, 2277–22803 Hannon, G.J. (2002) <strong>RNA</strong> <strong><strong>in</strong>terference</strong>. Nature 418, 244–2514 Plasterk, R.H.A. (2002) <strong>RNA</strong> silenc<strong>in</strong>g: <strong>the</strong> genome’s immune system.Science 296, 1263–12655 Zamore, P.D. (2002) Ancient pathways programmed by small <strong>RNA</strong>s.Science 296, 1265–12696 Zamore, P.D. et al. (2000) <strong>RNA</strong>i: double-str<strong>and</strong>ed <strong>RNA</strong> directs <strong>the</strong>ATP-dependent cleavage of m<strong>RNA</strong> at 21 to 23 nucleotide <strong>in</strong>tervals. Cell101, 25–337 Bernste<strong>in</strong>, E. et al. (2001) Role for a bidentate ribonuclease <strong>in</strong> <strong>the</strong><strong>in</strong>itiation step of <strong>RNA</strong> <strong><strong>in</strong>terference</strong>. Nature 409, 363–3668 Hammond, S.M. et al. (2000) An <strong>RNA</strong>-directed nuclease mediates posttranscriptionalgene silenc<strong>in</strong>g <strong>in</strong> Drosophila <strong>cell</strong>s. Nature 404,293–2969 Nykänen, A. et al. (2001) ATP requirements <strong>and</strong> small <strong>in</strong>terfer<strong>in</strong>g <strong>RNA</strong>structure <strong>in</strong> <strong>the</strong> <strong>RNA</strong> <strong><strong>in</strong>terference</strong> pathway. Cell 107, 309–32110 Mart<strong>in</strong>ez, J. et al. (2002) S<strong>in</strong>gle-str<strong>and</strong>ed antisense si<strong>RNA</strong>s guidetarget <strong>RNA</strong> cleavage <strong>in</strong> <strong>RNA</strong>i. Cell 110, 563–57411 Elbashir, S.M. et al. (2001) <strong>RNA</strong> <strong><strong>in</strong>terference</strong> is mediated by 21- <strong>and</strong> 22-nucleotide <strong>RNA</strong>s. Genes Dev. 15, 188–20012 Holen, T. et al. (2002) Positional effects of short <strong>in</strong>terfer<strong>in</strong>g <strong>RNA</strong>starget<strong>in</strong>g <strong>the</strong> human coagulation trigger tissue factor. Nucleic AcidsRes. 30, 1757–176613 Han, Y. <strong>and</strong> Grierson, D. (2002) Relationship between small antisense<strong>RNA</strong>s <strong>and</strong> aberrant <strong>RNA</strong>s associated with sense transgene mediatedgene silenc<strong>in</strong>g <strong>in</strong> tomato. Plant J. 29, 509–51914 Hammond, S.M. et al. (2001) Argonaute2, a l<strong>in</strong>k between genetic <strong>and</strong>biochemical analyses of <strong>RNA</strong>i. Science 293, 1146–115015 Bohmert, K. et al. (1998) AGO1 def<strong>in</strong>es a novel locus of Arabidopsiscontroll<strong>in</strong>g leaf development. EMBO J. 17, 170–18016 Tabara, H. et al. (1999) The rde-1 gene, <strong>RNA</strong> <strong><strong>in</strong>terference</strong>, <strong>and</strong>transposon silenc<strong>in</strong>g <strong>in</strong> C. elegans. Cell 99, 123–13217 Hutvágner, G. <strong>and</strong> Zamore, P.D. (2002) A micro<strong>RNA</strong> <strong>in</strong> a multipleturnover<strong>RNA</strong>i enzyme complex. Science 297, 2056–206018 Cerutti, L. et al. (2000) Doma<strong>in</strong>s <strong>in</strong> gene silenc<strong>in</strong>g <strong>and</strong> <strong>cell</strong>differentiation prote<strong>in</strong>s: <strong>the</strong> novel PAZ doma<strong>in</strong> <strong>and</strong> redef<strong>in</strong>ition of<strong>the</strong> Piwi doma<strong>in</strong>. Trends Biochem. Sci. 25, 481–48219 Tabara, H. et al. (2002) The ds<strong>RNA</strong> b<strong>in</strong>d<strong>in</strong>g prote<strong>in</strong> RDE-4 <strong>in</strong>teractswith RDE-1, DCR-1, <strong>and</strong> a DExH-box helicase to direct <strong>RNA</strong>i <strong>in</strong>C. elegans. Cell 109, 861–87120 Grishok, A. et al. (2000) Genetic requirements for <strong>in</strong>heritance of <strong>RNA</strong>i<strong>in</strong> C. elegans. Science 287, 2494–249721 Kett<strong>in</strong>g, R.F. et al. (1999) Mut-7 of C. elegans, required for transposonsilenc<strong>in</strong>g <strong>and</strong> <strong>RNA</strong> <strong><strong>in</strong>terference</strong>, is a homolog of Werner syndromehelicase <strong>and</strong> RNaseD. Cell 99, 133–14122 Kennerdell, J.R. <strong>and</strong> Car<strong>the</strong>w, R.W. (1998) Use of ds<strong>RNA</strong>-mediatedgenetic <strong><strong>in</strong>terference</strong> to demonstrate that frizzled <strong>and</strong> frizzled 2 act <strong>in</strong><strong>the</strong> w<strong>in</strong>gless pathway. Cell 95, 1017–102623 Svoboda, P. et al. (2000) Selective reduction of dormant maternalm<strong>RNA</strong>s <strong>in</strong> mouse oocytes by <strong>RNA</strong> <strong><strong>in</strong>terference</strong>. Development 127,4147–415624 Ratcliff, F. et al. (1997) A similarity between viral defense <strong>and</strong> genesilenc<strong>in</strong>g <strong>in</strong> plants. Science 276, 1558–156025 Bitko, V. <strong>and</strong> Barik, S. (2001) Phenotypic silenc<strong>in</strong>g of cytoplasmicgenes us<strong>in</strong>g sequence-specific double-str<strong>and</strong>ed short <strong>in</strong>terfer<strong>in</strong>g <strong>RNA</strong><strong>and</strong> its application <strong>in</strong> <strong>the</strong> reverse genetics of wild type negative-str<strong>and</strong><strong>RNA</strong> viruses. BMC Microbiol. 1, 34–4526 Williams, R.W. <strong>and</strong> Rub<strong>in</strong>, G.M. (2002) ARGONAUTE1 is required forefficient <strong>RNA</strong> <strong><strong>in</strong>terference</strong> <strong>in</strong> Drosophila embryos. Proc. Natl Acad.Sci. USA 99, 6889–689427 Kataoka, Y. et al. (2001) Developmental roles <strong>and</strong> molecularcharacterization of a Drosophila homologue of Arabidopsis Argonaute1,<strong>the</strong> founder of a novel gene superfamily. Genes Cells 6,313–32528 Harris, A.N. <strong>and</strong> Macdonald, P.M. (2001) auberg<strong>in</strong>e encodes aDrosophila polar granule component required for pole <strong>cell</strong> formation<strong>and</strong> related to eIF2C. Development 128, 2823–283229 Zeng, Y. <strong>and</strong> Cullen, B.R. (2002) <strong>RNA</strong> <strong><strong>in</strong>terference</strong> <strong>in</strong> human <strong>cell</strong>s isrestricted to <strong>the</strong> cytoplasm. <strong>RNA</strong> 8, 855–86030 Billy, E. et al. (2001) Specific <strong><strong>in</strong>terference</strong> with gene expression <strong>in</strong>ducedby long, double-str<strong>and</strong>ed <strong>RNA</strong> <strong>in</strong> mouse embryonal teratocarc<strong>in</strong>oma<strong>cell</strong> l<strong>in</strong>es. Proc. Natl Acad. Sci. USA 98, 14428–1443331 Cikaluk, D.E. et al. (1999) GERp95, a membrane-associated prote<strong>in</strong>that belongs to a family of prote<strong>in</strong>s <strong>in</strong>volved <strong>in</strong> stem <strong>cell</strong> differentiation.Mol. Biol. Cell 10, 3357–337232 Wesley, S.V. et al. (2001) Construct design for efficient, effective <strong>and</strong>high-throughput gene silenc<strong>in</strong>g <strong>in</strong> plants. Plant J. 27, 581–59033 Kalidas, S. <strong>and</strong> Smith, D.P. (2002) Novel genomic cDNA hybridshttp://tigs.trends.com
46Review TRENDS <strong>in</strong> Genetics Vol.19 No.1 January 2003produce effective <strong>RNA</strong> <strong><strong>in</strong>terference</strong> <strong>in</strong> adult Drosophila. Neuron 33,177–18434 Reed, R. <strong>and</strong> Hurt, E. (2002) A conserved m<strong>RNA</strong> export mach<strong>in</strong>erycoupled to pre-m<strong>RNA</strong> splic<strong>in</strong>g. Cell 108, 523–53135 Mette, M.F. et al. (2001) Resistance of <strong>RNA</strong>-mediated TGS to HC-Pro, aviral suppressor of PTGS, suggests alternative pathways for ds<strong>RNA</strong>process<strong>in</strong>g. Curr. Biol. 11, 1119 – 112336 Sijen, T. et al. (2001) On <strong>the</strong> role of <strong>RNA</strong> amplification <strong>in</strong> ds<strong>RNA</strong>triggeredgene silenc<strong>in</strong>g. Cell 107, 465–47637 Tijsterman, M. et al. (2002) <strong>RNA</strong> helicase MUT-14-dependent genesilenc<strong>in</strong>g triggered <strong>in</strong> C. elegans by short antisense <strong>RNA</strong>s. Science 295,694–69738 Dalmay, T. et al. (2000) An <strong>RNA</strong>-dependent <strong>RNA</strong> polymerase gene <strong>in</strong>Arabidopsis is required for posttranscriptional gene silenc<strong>in</strong>gmediated by a transgene but not by a virus. Cell 101, 543–55339 Mourra<strong>in</strong>, P. et al. (2000) Arabidopsis SGS2 <strong>and</strong> SGS3 genes arerequired for posttranscriptional gene silenc<strong>in</strong>g <strong>and</strong> natural virusresistance. Cell 101, 533–54240 Martens, H. et al. (2002) <strong>RNA</strong>i <strong>in</strong> Dictyostelium: <strong>the</strong> role of <strong>RNA</strong>directed<strong>RNA</strong> polymerases <strong>and</strong> double-str<strong>and</strong>ed RNase. Mol. Biol. Cell13, 445–45341 Lipardi, C. et al. (2001) <strong>RNA</strong>i as r<strong>and</strong>om degradative PCR: si<strong>RNA</strong>primers convert m<strong>RNA</strong> <strong>in</strong>to ds<strong>RNA</strong>s that are degraded to generatenew si<strong>RNA</strong>s. Cell 107, 297–30742 Bécl<strong>in</strong>, C. et al. (2002) A branched pathway for transgene-<strong>in</strong>duced<strong>RNA</strong> silenc<strong>in</strong>g <strong>in</strong> plants. Curr. Biol. 12, 684–68843 Vaistij, F.E. et al. (2002) Spread<strong>in</strong>g of <strong>RNA</strong> target<strong>in</strong>g <strong>and</strong> DNAmethylation <strong>in</strong> <strong>RNA</strong> silenc<strong>in</strong>g requires transcription of <strong>the</strong> target gene<strong>and</strong> a putative <strong>RNA</strong>-dependent <strong>RNA</strong> polymerase. Plant Cell 14,857–86744 Morel, J.-B. et al. (2000) DNA methylation <strong>and</strong> chromat<strong>in</strong> structureaffect transcriptional <strong>and</strong> post-transcriptional transgene silenc<strong>in</strong>g <strong>in</strong>Arabidopsis. Curr. Biol. 10, 1591–159445 Rountree, M.R. <strong>and</strong> Selker, E.U. (1997) DNA methylation <strong>in</strong>hibitselongation but not <strong>in</strong>itiation of transcription <strong>in</strong> Neurospora crassa.Genes Dev. 11, 2383–239546 Buhr, T. et al. (2002) Ribozyme term<strong>in</strong>ation of <strong>RNA</strong> transcripts downregulateseed fatty acid genes <strong>in</strong> transgenic soybean. Plant J. 30,155–16347 Pal-Bhadra, M. et al. (2002) <strong>RNA</strong>i related mechanisms affect bothtranscriptional <strong>and</strong> posttranscriptional transgene silenc<strong>in</strong>g <strong>in</strong> Drosophila.Mol. Cell 9, 315–32748 Cox, D.N. et al. (2000) piwi encodes a nucleoplasmic factor whoseactivity modulates <strong>the</strong> number <strong>and</strong> division rate of germl<strong>in</strong>e stem <strong>cell</strong>s.Development 127, 503–51449 Volpe, T.A. et al. (2002) Regulation of heterochromatic silenc<strong>in</strong>g <strong>and</strong>histone H3 lys<strong>in</strong>e-9 methylation by <strong>RNA</strong>i. Science 297, 1833–183750 Simmer, F. et al. (2002) Loss of <strong>the</strong> putative <strong>RNA</strong>-directed <strong>RNA</strong>polymerase RRF-3 makes C. elegans hypersensitive to <strong>RNA</strong>i. Curr.Biol. 12, 1317–131951 Montgomery, M.K. et al. (1998) <strong>RNA</strong> as a target of double-str<strong>and</strong>ed<strong>RNA</strong>-mediated genetic <strong><strong>in</strong>terference</strong> <strong>in</strong> Caenorhabditis elegans. Proc.Natl Acad. Sci. USA 95, 15502–1550752 Bosher, J.M. et al. (1999) <strong>RNA</strong> <strong><strong>in</strong>terference</strong> can target pre-m<strong>RNA</strong>:consequences for gene expression <strong>in</strong> a Caenorhabditis elegans operon.Genetics 153, 1245–125653 Wu-Scharf, D. et al. (2000) Transgene <strong>and</strong> transposon silenc<strong>in</strong>g <strong>in</strong>Chlamydomonas re<strong>in</strong>hardtii by a DEAH-box <strong>RNA</strong> helicase. Science290, 1159–116254 Waterhouse, P.M. et al. (2001) Gene silenc<strong>in</strong>g as an adaptive defenceaga<strong>in</strong>st viruses. Nature 411, 834–84255 Mourelatos, Z. et al. (2002) miRNPs: a novel class of ribonucleoprote<strong>in</strong>sconta<strong>in</strong><strong>in</strong>g numerous micro<strong>RNA</strong>s. Genes Dev. 16, 720–72856 Charroux, B. et al. (2000) Gem<strong>in</strong>4: a novel component of <strong>the</strong> SMNcomplex that is found <strong>in</strong> both gems <strong>and</strong> nucleoli. J. Cell Biol. 148,1177–118657 Llave, C. et al. (2002) Endogenous <strong>and</strong> silenc<strong>in</strong>g-associated small<strong>RNA</strong>s <strong>in</strong> plants. Plant Cell 14, 1605–161958 Hamilton, A. et al. (2002) Two classes of short <strong>in</strong>terfer<strong>in</strong>g <strong>RNA</strong> <strong>in</strong> <strong>RNA</strong>silenc<strong>in</strong>g. EMBO J. 21, 4671–467959 Re<strong>in</strong>hart, B.J. et al. (2002) Micro<strong>RNA</strong>s <strong>in</strong> plants. Genes Dev. 16,1616–162660 Jacobsen, S.E. et al. (1999) Disruption of an <strong>RNA</strong> helicase/<strong>RNA</strong>se IIIgene <strong>in</strong> Arabidopsis causes unregulated <strong>cell</strong> division <strong>in</strong> floralmeristems. Development 126, 5231–524361 Papaefthimiou, I. et al. (2001) Replicat<strong>in</strong>g potato sp<strong>in</strong>dle tuber viroid<strong>RNA</strong> is accompanied by short <strong>RNA</strong> fragments that are characteristic ofpost-transcriptional gene silenc<strong>in</strong>g. Nucleic Acids Res. 29, 2395–240062 Mallory, A.C. et al. (2001) HC-Pro suppression of transgene silenc<strong>in</strong>gelim<strong>in</strong>ates <strong>the</strong> small <strong>RNA</strong>s but not transgene methylation or <strong>the</strong>mobile signal. Plant Cell 13, 571–58363 Dudley, N.R. et al. (2002) Us<strong>in</strong>g <strong>RNA</strong> <strong><strong>in</strong>terference</strong> to identify genesrequired for <strong>RNA</strong> <strong><strong>in</strong>terference</strong>. Proc. Natl Acad. Sci. USA 99,4191–419664 Cogoni, C. <strong>and</strong> Mac<strong>in</strong>o, G. (1999) Posttranscriptional gene silenc<strong>in</strong>g <strong>in</strong>Neurospora by a RecQ DNA helicase. Science 286, 2342–234565 Re<strong>in</strong>hardt, B.J. <strong>and</strong> Bartel, D.P. (2002) Small <strong>RNA</strong>s correspond tocentromere heterochromatic repeats. Science 297, 183166 Hall, I.M. et al. (2002) Establishment <strong>and</strong> ma<strong>in</strong>tenance of aheterochromat<strong>in</strong> doma<strong>in</strong>. Science 297, 2232–223767 Mochizuki, K. et al. (2002) Analysis of a piwi-related gene implicatessmall <strong>RNA</strong>s <strong>in</strong> genome rearrangement <strong>in</strong> Tetrahymena. Cell 110,689–69968 Taverna, S.D. et al. (2002) Methylation of histone H3 at lys<strong>in</strong>e 9 targetsprogrammed DNA elim<strong>in</strong>ation <strong>in</strong> Tetrahymena. Cell 110, 701–71169 Lachner, M. <strong>and</strong> Jenuwe<strong>in</strong>, T. (2002) The many faces of histone lys<strong>in</strong>emethylation. Curr. Op<strong>in</strong>. Cell Biol. 14, 286–29870 Pasqu<strong>in</strong>elli, A.E. (2002) Micro<strong>RNA</strong>s: deviants no longer. Trends Genet.18, 171–17371 Rhoades, M.W. et al. (2002) Prediction of plant micro<strong>RNA</strong> targets. Cell110, 513–52072 Llave, C. et al. (2002) Cleavage of Scarecrow-like m<strong>RNA</strong> targetsdirected by a class of Arabidopsis mi<strong>RNA</strong>. Science 297, 2053–205673 Lee, Y. et al. (2002) Micro<strong>RNA</strong> maturation: stepwise process<strong>in</strong>g <strong>and</strong>sub<strong>cell</strong>ular localization. EMBO J. 21, 4663–467074 Grishok, A. et al. (2001) Genes <strong>and</strong> mechanisms related to <strong>RNA</strong><strong><strong>in</strong>terference</strong> regulate expression of <strong>the</strong> small temporal <strong>RNA</strong>s thatcontrol C. elegans developmental tim<strong>in</strong>g. Cell 106, 23–3475 Arav<strong>in</strong>, A.A. et al. (2001) Double-str<strong>and</strong>ed <strong>RNA</strong>-mediated silenc<strong>in</strong>gof genomic t<strong>and</strong>em repeats <strong>and</strong> transposable elements <strong>in</strong> <strong>the</strong>D. melanogaster germl<strong>in</strong>e. Curr. Biol. 11, 1017–1027http://tigs.trends.com