Chapter 7
Chapter 7
Chapter 7
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
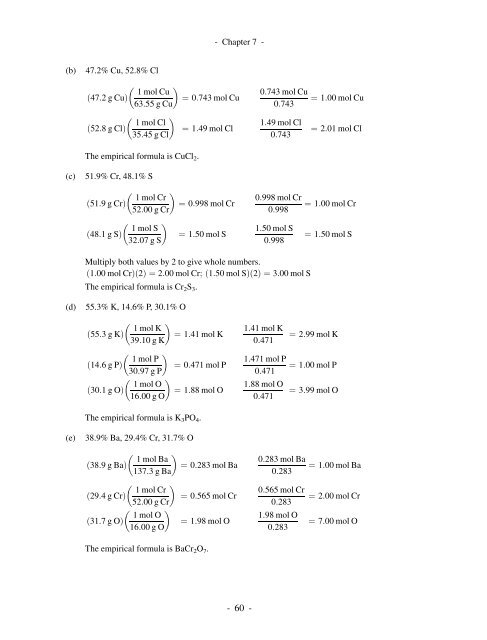
- <strong>Chapter</strong> 7 -(b)(c)47.2% Cu, 52.8% Cl 1 mol Cuð47:2gCuÞ¼ 0:743 mol Cu63:55 g Cu 1 mol Clð52:8gClÞ¼ 1:49 mol Cl35:45 g ClThe empirical formula is CuCl 2 .51.9% Cr, 48.1% S 1 mol Crð51:9gCrÞ¼ 0:998 mol Cr52:00 g Cr 1 mol Sð48:1gSÞ¼ 1:50 mol S32:07 g S0:743 mol Cu0:7431:49 mol Cl0:7430:998 mol Cr0:9981:50 mol S0:998¼ 1:00 mol Cu¼ 2:01 mol Cl¼ 1:00 mol Cr¼ 1:50 mol SMultiply both values by 2 to give whole numbers.ð1:00 mol CrÞðÞ¼2:00 2 mol Cr; ð1:50 mol SÞðÞ¼3:00 2 mol SThe empirical formula is Cr 2 S 3 .(d)(e)55.3% K, 14.6% P, 30.1% O 1 mol Kð55:3gKÞ¼ 1:41 mol K39:10 g K 1 mol Pð14:6gPÞ¼ 0:471 mol P30:97 g P 1 mol Oð30:1gOÞ¼ 1:88 mol O16:00 g OThe empirical formula is K 3 PO 4 .38.9% Ba, 29.4% Cr, 31.7% O 1 mol Bað38:9gBaÞ¼ 0:283 mol Ba137:3gBa 1 mol Crð29:4gCrÞ¼ 0:565 mol Cr52:00 g Cr 1 mol Oð31:7gOÞ¼ 1:98 mol O16:00 g OThe empirical formula is BaCr 2 O 7 .1:41 mol K0:4711:471 mol P0:4711:88 mol O0:4710:283 mol Ba0:2830:565 mol Cr0:2831:98 mol O0:283¼ 2:99 mol K¼ 1:00 mol P¼ 3:99 mol O¼ 1:00 mol Ba¼ 2:00 mol Cr¼ 7:00 mol O-60-