Chapter 7
Chapter 7
Chapter 7
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
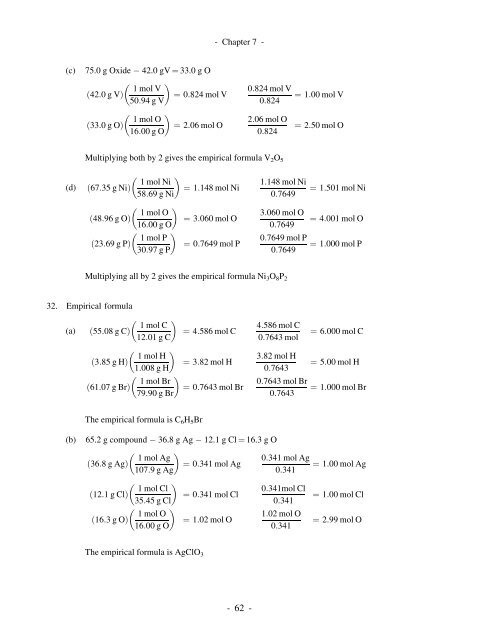
- <strong>Chapter</strong> 7 -(c) 75.0 g Oxide 42.0 gV ¼ 33.0 g O 1 mol Vð42:0gVÞ¼ 0:824 mol V50:94 g V 1 mol Oð33:0gOÞ¼ 2:06 mol O16:00 g O0:824 mol V0:8242:06 mol O0:824¼ 1:00 mol V¼ 2:50 mol OMultiplying both by 2 gives the empirical formula V 2 O 5(d) 1 mol Nið67:35 g NiÞ58:69 g Ni 1 mol Oð48:96 g OÞ16:00 g Oð23:69 g PÞ1 mol P30:97 g P¼ 1:148 mol Ni¼ 3:060 mol O¼ 0:7649 mol P1:148 mol Ni0:76493:060 mol O0:76490:7649 mol P0:7649¼ 1:501 mol Ni¼ 4:001 mol O¼ 1:000 mol PMultiplying all by 2 gives the empirical formula Ni 3 O 8 P 232. Empirical formula 1 mol C(a) ð55:08 g CÞ¼ 4:586 mol C12:01 g C 1 mol Hð3:85 g HÞ¼ 3:82 mol H1:008 g H 1 mol Brð61:07 g BrÞ¼ 0:7643 mol Br79:90 g Br4:586 mol C0:7643 mol3:82 mol H0:76430:7643 mol Br0:7643¼ 6:000 mol C¼ 5:00 mol H¼ 1:000 mol BrThe empirical formula is C 6 H 5 Br(b) 65.2 g compound 36.8 g Ag 12.1 g Cl ¼ 16.3 g O 1 mol Ag0:341 mol Agð36:8gAgÞ¼ 0:341 mol Ag¼ 1:00 mol Ag107:9gAg0:341 1 mol Cl0:341mol Clð12:1gClÞ¼ 0:341 mol Cl¼ 1:00 mol Cl35:45 g Cl0:341 1 mol O1:02 mol Oð16:3gOÞ¼ 1:02 mol O¼ 2:99 mol O16:00 g O0:341The empirical formula is AgClO 3-62-