Core-Shell
Core-Shell
Core-Shell
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
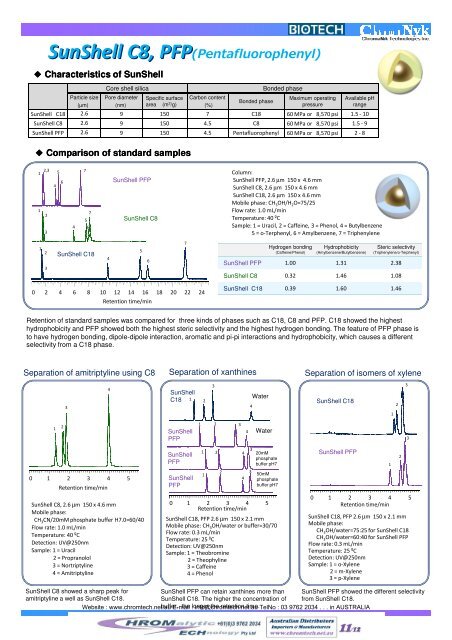
Particle size(µm)<strong>Core</strong> shell silicaPore diameter(nm)Specific surfacearea (m 2 /g)Sun<strong>Shell</strong> の 基 本 特 性Sun<strong>Shell</strong> C8, PFP(Pentafluorophenyl)PFP◆ Characteristics of Sun<strong>Shell</strong>Carbon content(%)Bonded phaseBonded phaseMaximum operatingpressureAvailable pHrangeSun<strong>Shell</strong> C18 2.6 9 150 7 C18 60 MPa or 8,570 psi 1.5 - 10Sun<strong>Shell</strong> C8 2.6 9 150 4.5 C8 60 MPa or 8,570 psi 1.5 - 9Sun<strong>Shell</strong> PFP 2.6 9 150 4.5 Pentafluorophenyl 60 MPa or 8,570 psi 2 - 8◆ Comparison of standard samples112,32356447756Sun<strong>Shell</strong> PFPSun<strong>Shell</strong> C8Column:Sun<strong>Shell</strong> PFP, 2.6 µm 150 x 4.6 mmSun<strong>Shell</strong> C8, 2.6 µm 150 x 4.6 mmSun<strong>Shell</strong> C18, 2.6 µm 150 x 4.6 mmMobile phase: CH 3 OH/H 2 O=75/25Flow rate: 1.0 mL/minTemperature: 40 ºCSample: 1 = Uracil, 2 = Caffeine, 3 = Phenol, 4 = Butylbenzene5 = o-Terphenyl, 6 = Amylbenzene, 7 = Triphenylene123Sun<strong>Shell</strong> C184567Hydrogen bonding(Caffeine/Phenol)Hydrophobicity(Amylbenzene/Butylbenzene)Steric selectivity(Triphenylene/o-Terphenyl)Sun<strong>Shell</strong> PFP 1.00 1.31 2.38Sun<strong>Shell</strong> C8 0.32 1.46 1.080 2 4 6 8 10 12 14 16 18 20 22 24Retention time/minSun<strong>Shell</strong> C18 0.39 1.60 1.46Retention of standard samples was compared for three kinds of phases such as C18, C8 and PFP. C18 showed the highesthydrophobicity and PFP showed both the highest steric selectivity and the highest hydrogen bonding. The feature of PFP phase isto have hydrogen bonding, dipole-dipole interaction, aromatic and pi-pi interactions and hydrophobicity, which causes a differentselectivity from a C18 phase.Separation of amitriptyline using C8 Separation of xanthines Separation of isomers of xylene34Sun<strong>Shell</strong>C181 234WaterSun<strong>Shell</strong> C1812312Sun<strong>Shell</strong>PFP1 2 34Water30 1 2 3 4 5Retention time/minSun<strong>Shell</strong> C8, 2.6 µm 150 x 4.6 mmMobile phase:CH 3 CN/20mM phosphate buffer H7.0=60/40Flow rate: 1.0 mL/minTemperature: 40 ºCDetection: UV@250nmSample: 1 = Uracil2 = Propranolol3 = Nortriptyline4 = AmitriptylineSun<strong>Shell</strong>PFPSun<strong>Shell</strong>PFP1 2120mMphosphatebuffer pH750mMphosphatebuffer pH70 1 2 3 4 5Retention time/minSun<strong>Shell</strong> C18, PFP 2.6 µm 150 x 2.1 mmMobile phase: CH 3 OH/water or buffer=30/70Flow rate: 0.3 mL/minTemperature: 25 ºCDetection: UV@250nmSample: 1 = Theobromine2 = Theophyline3 = Caffeine4 = Phenol42 343Sun<strong>Shell</strong> PFP0 1 2 3 4 5Retention time/minSun<strong>Shell</strong> C18, PFP 2.6 µm 150 x 2.1 mmMobile phase:CH 3 OH/water=75:25 for Sun<strong>Shell</strong> C18CH 3 OH/water=60:40 for Sun<strong>Shell</strong> PFPFlow rate: 0.3 mL/minTemperature: 25 ºCDetection: UV@250nmSample: 1 = o-Xylene2 = m-Xylene3 = p-Xylene12Sun<strong>Shell</strong> C8 showed a sharp peak foramitriptyline a well as Sun<strong>Shell</strong> C18.Sun<strong>Shell</strong> PFP can retain xanthines more thanSun<strong>Shell</strong> C18. The higher the concentration ofSun<strong>Shell</strong> PFP showed the different selectivityfrom Sun<strong>Shell</strong> C18.Website : www.chromtech.net.au buffer, E-mail the : longer info@chromtech.net.au the retention time. TelNo : 03 9762 2034 . . . in AUSTRALIA