- Page 1 and 2: FINAL REPORTEnhanced Reactant-Conta
- Page 3 and 4: 2. FRONT MATTERTable of Contents1.
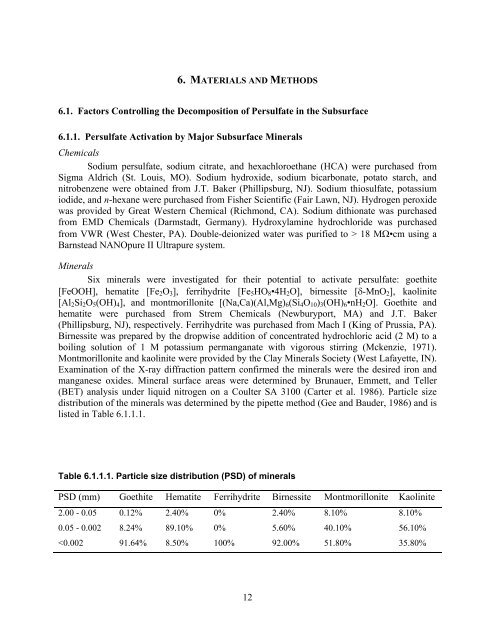
- Page 5 and 6: 7.1.1. Persulfate Activation by Maj
- Page 7 and 8: List of TablesTable 5.1. Second ord
- Page 9 and 10: Figure 7.1.2.7. Degradation of the
- Page 11 and 12: Base:persulfate ratio of 2:1; c) Ba
- Page 13 and 14: nitrobenzene; 15 mL total volume. E
- Page 15 and 16: Figure 7.2.7.5. CB degradation in b
- Page 17 and 18: Figure 7.3.1.31. Persulfate concent
- Page 19 and 20: List of AcronymsBETBTEXCBCECCHPCTDC
- Page 21 and 22: KeywordsIn situ chemical oxidation,
- Page 23 and 24: 4. OBJECTIVEThe goal of research co
- Page 25 and 26: strong (E 0 = 2.1 V), water-soluble
- Page 27 and 28: Persulfate also has the unique prop
- Page 29 and 30: enzenes react with sulfate radicals
- Page 31 and 32: tomography (XRCT) is a non-destruct
- Page 33: The diffusion of a remedial agent t
- Page 37 and 38: 6.1.2. Persulfate Activation by Sub
- Page 39 and 40: 6.1.3. Base-Activated Persulfate Tr
- Page 41 and 42: 6.2. Persulfate Reactivity Under Di
- Page 43 and 44: temperatures were 220˚C and 270˚C
- Page 45 and 46: time point and were capped to minim
- Page 47 and 48: form of hydroperoxide. Control reac
- Page 49 and 50: 6.2.4. Persulfate Activation By Phe
- Page 51 and 52: °C, detector temperature of 270 °
- Page 53 and 54: Analytical ProceduresHexane extract
- Page 55 and 56: Soil organic matter (SOM) was remov
- Page 57 and 58: 6.2.8. Effect of Sorption on Contam
- Page 59 and 60: 6.3. Effect of Persulfate on Subsur
- Page 61 and 62: Table 6.3.2.2. Chemical properties
- Page 63 and 64: Figure 6.3.2.1. Falling head permea
- Page 65 and 66: StartPrepareilImage generationandre
- Page 67 and 68: cure for 24 hr. The Palouse loess w
- Page 69 and 70: 7. RESULTS AND ACCOMPLISHMENTS7.1.
- Page 71 and 72: Table 7.1.1.1. Persulfate decomposi
- Page 73 and 74: aControlPositive controlFerrihydrit
- Page 75 and 76: aControlPositive controlFerrihydrit
- Page 77 and 78: a1Nitrobenzene, C/C 00.80.60.40.20C
- Page 79 and 80: a1Hexachloroethane, C/C 00.80.60.40
- Page 81 and 82: Table 7.1.1.4. Persulfate decomposi
- Page 83 and 84: Table 7.1.1.5. Nitrobenzene decompo
- Page 85 and 86:
Table 7.1.1.6. HCA decomposition ra
- Page 87 and 88:
aControlPositive control0.03 g Goet
- Page 89 and 90:
7.1.2. Persulfate Activation by Sub
- Page 91 and 92:
a1b1Nitrobenzene remaining after 48
- Page 93 and 94:
a1Anisole (mM)0.80.60.40.2Unactivat
- Page 95 and 96:
aNitrobenzene (mM)10.80.60.40.20Una
- Page 97 and 98:
a1, 3, 5-Trinitrobenzene (mM)10.80.
- Page 99 and 100:
All of the mineral persulfate syste
- Page 101 and 102:
7.1.3. Base-Activated Persulfate Tr
- Page 103 and 104:
1412KB1KB2108pH64200 1 2 3 4 5 6Tim
- Page 105 and 106:
a0.5Persulfate Concentration (M)0.4
- Page 107 and 108:
aPersulfate Concentration (M)0.50.4
- Page 109 and 110:
pH Drift in Soil SlurriespH was mon
- Page 111 and 112:
a1412108pH6420pH 12pH 10pH 80 3 6 9
- Page 113 and 114:
a141210pH8642pH 12pH 10pH 800 1 2 3
- Page 115 and 116:
aNitrobenzene Concentration (C/C o)
- Page 117 and 118:
Superoxide Radical and Reductant Ge
- Page 119 and 120:
aHexachloroethane Concentration (C/
- Page 121 and 122:
7.1.4. Summary of Factors Controlli
- Page 123 and 124:
Figure 7.2.1.1. Persulfate decompos
- Page 125 and 126:
Figure 7.2.1.2. Degradation of the
- Page 127 and 128:
adical and hydroxyl radical. A simi
- Page 129 and 130:
Detection of Reactive Oxygen Specie
- Page 131 and 132:
Figure 7.2.1.7. Degradation of the
- Page 133 and 134:
7.2.2. Effect of Basicity on Persul
- Page 135 and 136:
The mechanism of base-activated per
- Page 137 and 138:
Effect of Persulfate Concentration
- Page 139 and 140:
Relative rates of reactive oxygen s
- Page 141 and 142:
Relative rates of reductant generat
- Page 143 and 144:
ConclusionThe reactive species gene
- Page 145 and 146:
abFigure 7.2.3.1. a) First order de
- Page 147 and 148:
Figure 7.2.3.3. Effect of ionic str
- Page 149 and 150:
persulfate. Persulfate decomposes r
- Page 151 and 152:
Figure 7.2.3.6. Stoichiometry for t
- Page 153 and 154:
Figure 7.2.3.8. Effect of copper (I
- Page 155 and 156:
2 S 2 O 82-→ O 2 (7.2.3.9)To inve
- Page 157 and 158:
7.2.4. Persulfate Activation By Phe
- Page 159 and 160:
hexachloroethane, with pentachlorop
- Page 161 and 162:
1Anisole (C/C 0)0.80.60.4ControlPos
- Page 163 and 164:
a.Nitrobenzene (C/C o)10.80.60.4Con
- Page 165 and 166:
Mechanism of Persulfate ActivationT
- Page 167 and 168:
Degradation of pentachlorophenoxide
- Page 169 and 170:
Hydroxyl radical generation in the
- Page 171 and 172:
CompoundSuccinicAcidMolecularFormul
- Page 173 and 174:
The generation of hydroxyl radicals
- Page 175 and 176:
Table 7.2.5.2. Isomers of the alcoh
- Page 177 and 178:
a. b.Control, w/o alcoholn-Pentanol
- Page 179 and 180:
The results of Figures 7.2.5.5 and
- Page 181 and 182:
Control, w/o aldehydeFormaldehydeAc
- Page 183 and 184:
a. b.11Nitrobenzene (C/C 0)0.80.60.
- Page 185 and 186:
Control (Deionized water)Positive C
- Page 187 and 188:
Control (Deionized water)Positive C
- Page 189 and 190:
1Nitrobenzene (C/C o)0.80.60.40.2Co
- Page 191 and 192:
1Nitrobenzene (C/C o)0.80.60.40.2Co
- Page 193 and 194:
Control (Deionized water)Positive C
- Page 195 and 196:
Control (Deionized water)Positive C
- Page 197 and 198:
1Hexachloroethane (C/C o)0.80.60.40
- Page 199 and 200:
1Hexachloroethane (C/C o)0.80.60.40
- Page 201 and 202:
ConclusionThe results of this resea
- Page 203 and 204:
1PCE (C/C 0)0.80.60.40.2Control1:12
- Page 205 and 206:
Aromatic ContaminantsThe degradatio
- Page 207 and 208:
ConclusionThe results of this resea
- Page 209 and 210:
Activated Persulfate Treatment of S
- Page 211 and 212:
Figure 7.2.8.4. CHP treatment of HC
- Page 213 and 214:
Figure 7.2.8.6. Base-activated pers
- Page 215 and 216:
7.2.9. Summary of Persulfate Activa
- Page 217 and 218:
hematite, and goethite. However, pH
- Page 219 and 220:
Figure 7.3.1.2. XRD spectra for goe
- Page 221 and 222:
Figure 7.3.1.4. XRD spectra for goe
- Page 223 and 224:
Figure 7.3.1.6. XRD spectra for hem
- Page 225 and 226:
Figure 7.3.1.8. XRD spectra for hem
- Page 227 and 228:
Figure 7.3.1.10. XRD spectra for fe
- Page 229 and 230:
Figure 7.3.1.12. XRD spectra for fe
- Page 231 and 232:
Figure 7.3.1.14. XRD spectra for bi
- Page 233 and 234:
Figure 7.3.1.16. XRD spectra for bi
- Page 235 and 236:
Figure 7.3.1.18. XRD spectra for ka
- Page 237 and 238:
Figure 7.3.1.20. XRD spectra for ka
- Page 239 and 240:
Figure 7.3.1.22. XRD spectra for mo
- Page 241 and 242:
Figure 7.3.1.24. XRD spectra for mo
- Page 243 and 244:
1Persulfate (C/C 0)0.80.60.40.2Unac
- Page 245 and 246:
1Persulfate (C/C 0)0.80.60.40.2Unac
- Page 247 and 248:
1Persulfate (C/C 0)0.80.60.40.2Unac
- Page 249 and 250:
141210pH86Unactivated persulfateIro
- Page 251 and 252:
141210pH86Unactivated persulfateIro
- Page 253 and 254:
141210pH86Unactivated persulfateIro
- Page 255 and 256:
1412pH1086Unactivated persulfateIro
- Page 257 and 258:
7.3.2. Effect of Persulfate Formula
- Page 259 and 260:
Persulfate AlonePersulfate + Iron (
- Page 261 and 262:
Persulfate AlonePersulfate + Iron (
- Page 263 and 264:
with deionized water was 0.16. Thes
- Page 265 and 266:
hydraulic conductivity. These chang
- Page 267 and 268:
7.4. Transport of Persulfate into L
- Page 269 and 270:
0.1 M Persulfate Diffusion in Palou
- Page 271 and 272:
Persulfate concentration (M)0 0.2 0
- Page 273 and 274:
However, the concentration of base-
- Page 275 and 276:
Numerous phenoxides, including chlo
- Page 277 and 278:
9. LITERATURE CITEDAfanas’ev, A.M
- Page 279 and 280:
Crooks, V.E., Quigley, R.M. 1984. S
- Page 281 and 282:
Huang, K.C., Zhao, Z., Hoag, G.E.,
- Page 283 and 284:
Lunenok-Burmakina, V.A., Aleeva, G.
- Page 285 and 286:
Peyton, G.P. 1993. The free-radical
- Page 287 and 288:
Todres, Z.V. 2003. Organic ion radi
- Page 289 and 290:
10. APPENDICESList of Technical Pub