Performance & Molecular Structure of Fuel Oils & Lube Base Oils ...
Performance & Molecular Structure of Fuel Oils & Lube Base Oils ...
Performance & Molecular Structure of Fuel Oils & Lube Base Oils ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
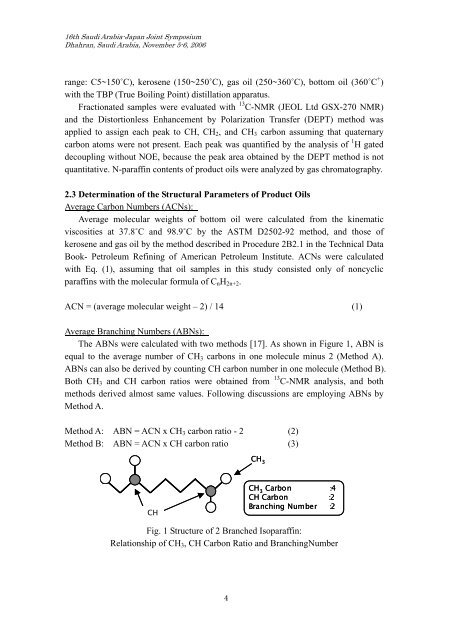
16th Saudi Arabia-Japan Joint SymposiumDhahran, Saudi Arabia, November 5-6, 2006range: C5~150˚C), kerosene (150~250˚C), gas oil (250~360˚C), bottom oil (360˚C + )with the TBP (True Boiling Point) distillation apparatus.Fractionated samples were evaluated with 13 C-NMR (JEOL Ltd GSX-270 NMR)and the Distortionless Enhancement by Polarization Transfer (DEPT) method wasapplied to assign each peak to CH, CH 2 , and CH 3 carbon assuming that quaternarycarbon atoms were not present. Each peak was quantified by the analysis <strong>of</strong> 1 H gateddecoupling without NOE, because the peak area obtained by the DEPT method is notquantitative. N-paraffin contents <strong>of</strong> product oils were analyzed by gas chromatography.2.3 Determination <strong>of</strong> the Structural Parameters <strong>of</strong> Product <strong>Oils</strong>Average Carbon Numbers (ACNs):Average molecular weights <strong>of</strong> bottom oil were calculated from the kinematicviscosities at 37.8˚C and 98.9˚C by the ASTM D2502-92 method, and those <strong>of</strong>kerosene and gas oil by the method described in Procedure 2B2.1 in the Technical DataBook- Petroleum Refining <strong>of</strong> American Petroleum Institute. ACNs were calculatedwith Eq. (1), assuming that oil samples in this study consisted only <strong>of</strong> noncyclicparaffins with the molecular formula <strong>of</strong> C n H 2n+2 .ACN = (average molecular weight – 2) / 14 (1)Average Branching Numbers (ABNs):The ABNs were calculated with two methods [17]. As shown in Figure 1, ABN isequal to the average number <strong>of</strong> CH 3 carbons in one molecule minus 2 (Method A).ABNs can also be derived by counting CH carbon number in one molecule (Method B).Both CH 3 and CH carbon ratios were obtained from 13 C-NMR analysis, and bothmethods derived almost same values. Following discussions are employing ABNs byMethod A.Method A: ABN = ACN x CH 3 carbon ratio - 2 (2)Method B: ABN = ACN x CH carbon ratio (3)CH 3CHCH 3 Carbon :4CH Carbon :2Branching Number :2Fig. 1 <strong>Structure</strong> <strong>of</strong> 2 Branched Isoparaffin:Relationship <strong>of</strong> CH 3 , CH Carbon Ratio and BranchingNumber4