Fuel Cell Systems Explained - from and for SET students
Fuel Cell Systems Explained - from and for SET students
Fuel Cell Systems Explained - from and for SET students
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
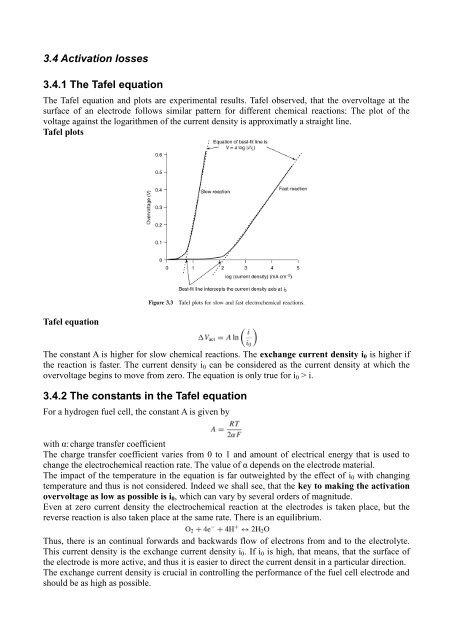
3.4 Activation losses3.4.1 The Tafel equationThe Tafel equation <strong>and</strong> plots are experimental results. Tafel observed, that the overvoltage at thesurface of an electrode follows similar pattern <strong>for</strong> different chemical reactions: The plot of thevoltage against the logarithmen of the current density is approximatly a straight line.Tafel plotsTafel equationThe constant A is higher <strong>for</strong> slow chemical reactions. The exchange current density i 0 is higher ifthe reaction is faster. The current density i 0 can be considered as the current density at which theovervoltage begins to move <strong>from</strong> zero. The equation is only true <strong>for</strong> i 0 > i.3.4.2 The constants in the Tafel equationFor a hydrogen fuel cell, the constant A is given bywith α: charge transfer coefficientThe charge transfer coefficient varies <strong>from</strong> 0 to 1 <strong>and</strong> amount of electrical energy that is used tochange the electrochemical reaction rate. The value of α depends on the electrode material.The impact of the temperature in the equation is far outweighted by the effect of i 0 with changingtemperature <strong>and</strong> thus is not considered. Indeed we shall see, that the key to making the activationovervoltage as low as possible is i 0 , which can vary by several orders of magnitude.Even at zero current density the electrochemical reaction at the electrodes is taken place, but thereverse reaction is also taken place at the same rate. There is an equilibrium.Thus, there is an continual <strong>for</strong>wards <strong>and</strong> backwards flow of electrons <strong>from</strong> <strong>and</strong> to the electrolyte.This current density is the exchange current density i 0 . If i 0 is high, that means, that the surface ofthe electrode is more active, <strong>and</strong> thus it is easier to direct the current densit in a particular direction.The exchange current density is crucial in controlling the per<strong>for</strong>mance of the fuel cell electrode <strong>and</strong>should be as high as possible.