Fuel Indexes: A Novel Method for the Evaluation of Relevant ...
Fuel Indexes: A Novel Method for the Evaluation of Relevant ...
Fuel Indexes: A Novel Method for the Evaluation of Relevant ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
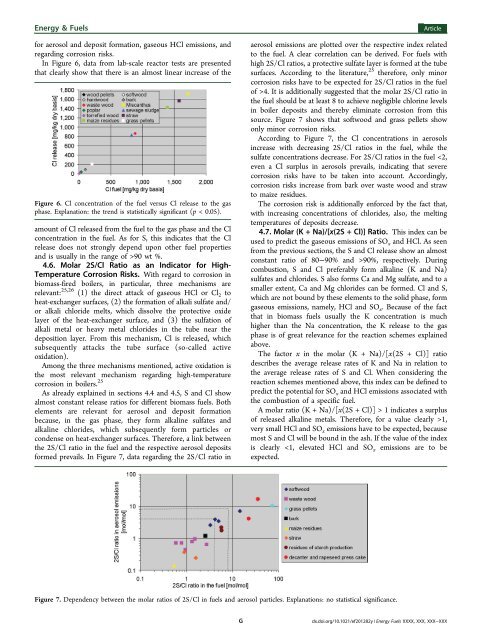
Energy & <strong>Fuel</strong>s<strong>for</strong> aerosol and deposit <strong>for</strong>mation, gaseous HCl emissions, andregarding corrosion risks.In Figure 6, data from lab-scale reactor tests are presentedthat clearly show that <strong>the</strong>re is an almost linear increase <strong>of</strong> <strong>the</strong>Figure 6. Cl concentration <strong>of</strong> <strong>the</strong> fuel versus Cl release to <strong>the</strong> gasphase. Explanation: <strong>the</strong> trend is statistically significant (p < 0.05).amount <strong>of</strong> Cl released from <strong>the</strong> fuel to <strong>the</strong> gas phase and <strong>the</strong> Clconcentration in <strong>the</strong> fuel. As <strong>for</strong> S, this indicates that <strong>the</strong> Clrelease does not strongly depend upon o<strong>the</strong>r fuel propertiesand is usually in <strong>the</strong> range <strong>of</strong> >90 wt %.4.6. Molar 2S/Cl Ratio as an Indicator <strong>for</strong> High-Temperature Corrosion Risks. With regard to corrosion inbiomass-fired boilers, in particular, three mechanisms arerelevant: 25,26 (1) <strong>the</strong> direct attack <strong>of</strong> gaseous HCl or Cl 2 toheat-exchanger surfaces, (2) <strong>the</strong> <strong>for</strong>mation <strong>of</strong> alkali sulfate and/or alkali chloride melts, which dissolve <strong>the</strong> protective oxidelayer <strong>of</strong> <strong>the</strong> heat-exchanger surface, and (3) <strong>the</strong> sulfation <strong>of</strong>alkali metal or heavy metal chlorides in <strong>the</strong> tube near <strong>the</strong>deposition layer. From this mechanism, Cl is released, whichsubsequently attacks <strong>the</strong> tube surface (so-called activeoxidation).Among <strong>the</strong> three mechanisms mentioned, active oxidation is<strong>the</strong> most relevant mechanism regarding high-temperaturecorrosion in boilers. 25As already explained in sections 4.4 and 4.5, S and Cl showalmost constant release ratios <strong>for</strong> different biomass fuels. Bo<strong>the</strong>lements are relevant <strong>for</strong> aerosol and deposit <strong>for</strong>mationbecause, in <strong>the</strong> gas phase, <strong>the</strong>y <strong>for</strong>m alkaline sulfates andalkaline chlorides, which subsequently <strong>for</strong>m particles orcondense on heat-exchanger surfaces. There<strong>for</strong>e, a link between<strong>the</strong> 2S/Cl ratio in <strong>the</strong> fuel and <strong>the</strong> respective aerosol deposits<strong>for</strong>med prevails. In Figure 7, data regarding <strong>the</strong> 2S/Cl ratio inArticleaerosol emissions are plotted over <strong>the</strong> respective index relatedto <strong>the</strong> fuel. A clear correlation can be derived. For fuels withhigh 2S/Cl ratios, a protective sulfate layer is <strong>for</strong>med at <strong>the</strong> tubesurfaces. According to <strong>the</strong> literature, 25 <strong>the</strong>re<strong>for</strong>e, only minorcorrosion risks have to be expected <strong>for</strong> 2S/Cl ratios in <strong>the</strong> fuel<strong>of</strong> >4. It is additionally suggested that <strong>the</strong> molar 2S/Cl ratio in<strong>the</strong> fuel should be at least 8 to achieve negligible chlorine levelsin boiler deposits and <strong>the</strong>reby eliminate corrosion from thissource. Figure 7 shows that s<strong>of</strong>twood and grass pellets showonly minor corrosion risks.According to Figure 7, <strong>the</strong> Cl concentrations in aerosolsincrease with decreasing 2S/Cl ratios in <strong>the</strong> fuel, while <strong>the</strong>sulfate concentrations decrease. For 2S/Cl ratios in <strong>the</strong> fuel 90%, respectively. Duringcombustion, S and Cl preferably <strong>for</strong>m alkaline (K and Na)sulfates and chlorides. S also <strong>for</strong>ms Ca and Mg sulfate, and to asmaller extent, Ca and Mg chlorides can be <strong>for</strong>med. Cl and S,which are not bound by <strong>the</strong>se elements to <strong>the</strong> solid phase, <strong>for</strong>mgaseous emissions, namely, HCl and SO x . Because <strong>of</strong> <strong>the</strong> factthat in biomass fuels usually <strong>the</strong> K concentration is muchhigher than <strong>the</strong> Na concentration, <strong>the</strong> K release to <strong>the</strong> gasphase is <strong>of</strong> great relevance <strong>for</strong> <strong>the</strong> reaction schemes explainedabove.The factor x in <strong>the</strong> molar (K + Na)/[x(2S + Cl)] ratiodescribes <strong>the</strong> average release rates <strong>of</strong> K and Na in relation to<strong>the</strong> average release rates <strong>of</strong> S and Cl. When considering <strong>the</strong>reaction schemes mentioned above, this index can be defined topredict <strong>the</strong> potential <strong>for</strong> SO x and HCl emissions associated with<strong>the</strong> combustion <strong>of</strong> a specific fuel.A molar ratio (K + Na)/[x(2S + Cl)] > 1 indicates a surplus<strong>of</strong> released alkaline metals. There<strong>for</strong>e, <strong>for</strong> a value clearly >1,very small HCl and SO x emissions have to be expected, becausemost S and Cl will be bound in <strong>the</strong> ash. If <strong>the</strong> value <strong>of</strong> <strong>the</strong> indexis clearly