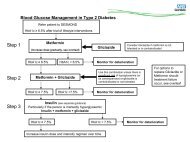
Prescribing Guidance for NOACs - NHS Cumbria
Prescribing Guidance for NOACs - NHS Cumbria
Prescribing Guidance for NOACs - NHS Cumbria
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
FURTHER PRESCRIBER GUIDANCE FOR DABIGATRAN (Pradaxa ).Interactions with other medicines and other relevant interactionsAnticoagulants and antiplatelets - concomitant use of other oral or parenteral anticoagulants increases majorbleeding rates with both dabigatran etexilate and warfarin by approximately 2.5-fold.NSAIDs - due to the risk of haemorrhage, notably with NSAIDs with elimination half-lives > 12 hours, closeobservation <strong>for</strong> signs of bleeding is recommended.Concomitant administration of strong P-gp inhibitors (such as amiodarone, verapamil, quinidine, ketoconazoleand clarithromycin) is expected to result in increased dabigatran plasma concentrations. If not otherwisespecifically described, close clinical surveillance (looking <strong>for</strong> signs of bleeding or anaemia) is required whendabigatran is co-administered with strong P-gp inhibitors.Close monitoring should be exercised when dabigatran etexilate is combined with clarithromycin and particularlyin the occurrence of bleeding, notably in patients having a mild to moderate renal impairment.Itraconazole, tacrolimus and cyclosporine are contra-indicated.In Inadequate case of intolerability clinical data to is dabigatran, available regarding patients should the co-administration be instructed to of immediately dabigatran consult and dronedarone their treating and their coadministrationorder is not to be recommended.switched to alternate acceptable treatment options <strong>for</strong> prevention of stroke andphysiciansystemic Concomitant embolism administration (SEE) associated of a P-gp with inducer atrial (such fibrillation. as rifampicin, St. John´s wort (Hypericum per<strong>for</strong>atum),carbamazepine, or phenytoin) is expected to result in decreased dabigatran concentrations and should beavoided.PatientsProteasebetweeninhibitors75-80includingyearsritonavirshould beandtreatedits combinationswith 150 mgwithcapsuleothertwiceproteasedaily.inhibitorsA doseaffectof 110P-gpmg capsule(either astwiceinhibitordailyorcanas inducer)be individuallyhave notconsidered,been studiedat theanddiscretionare there<strong>for</strong>eof thenotphysician,recommendedwhen the<strong>for</strong>thromboembolicconcomitant treatmentrisk islowwithanddabigatran.the bleeding risk is high.SSRIs and SNRIs increased the risk of bleeding in RE-LY in all treatment groups.When clinically relevant bleeding occurs, treatment should be interrupted. Bleeding can occur at any site duringtherapy with dabigatran. An unexplained fall in haemoglobin and/or haematocrit or blood pressure should lead toa search <strong>for</strong> a bleeding site.For subjects with gastritis, oesophagitis, or gastroesophageal reflux, the dose of 110 mg capsule twice daily maybe considered due to the elevated risk of major gastro-intestinal bleeding with dabigatran. As with warfarin, coadministrationof aspirin, clopidogrel and NSAID increases risk of bleeding.The administration of a PPI can be considered to help prevent GI bleeding.Patients with elevated liver enzymes > 2 upper limit of normal were excluded in the study investigating theprevention of stroke and systemic embolism associated with atrial fibrillation. No treatment experience isavailable <strong>for</strong> this subpopulation of patients and there<strong>for</strong>e the use of dabigatran is not recommended in thispopulation.A <strong>for</strong>gotten dose may still be taken up to 6 hours prior to the next scheduled dose. From 6 hours onwards prior tothe next scheduled dose, the missed dose should be omitted. No double dose should be taken to make up <strong>for</strong> amissed dose.Caution - Concomitant administration of strong P-gp inhibitors (such as amiodarone, verapamil, quinidine,ketoconazole and clarithromycin) is expected to result in increased dabigatran plasma concentrations andpotential increase in bleeding risk.
FURTHER PRESCRIBER GUIDANCE FOR RIVAROXABAN (Xarelto )Interactions with other medicines and other relevant interactionsThe use of rivaroxaban is not recommended in patients receiving concomitant systemic treatment with azoleantimycoticssuch as ketoconazole, itraconazole, voriconazole and posaconazole or HIV protease inhibitors.These active substances are strong inhibitors of both CYP3A4 and P-gp.Interactions with Clarithromycin, erythromycin and fluconazole are not considered clinically significant (SPC).Given the limited clinical data available with dronedarone, co-administration with rivaroxaban should beavoided.Care is to be taken if patients are treated concomitantly with NSAIDs (including acetylsalicylic acid) andplatelet aggregation inhibitors because these medicinal products typically increase the bleeding risk..No pharmacokinetic interaction was observed between warfarin and rivaroxaban.The concomitant use of rivaroxaban with other strong CYP3A4 inducers (e.g. phenytoin, carbamazepine,phenobarbital or St. John's Wort) may lead to reduced rivaroxaban plasma concentrations. Strong CYP3A4inducers should be co-administered with caution.In case of intolerability to rivaroxaban, patients should be instructed to immediately consult their treatingphysician in order to be switched to alternate acceptable treatment options <strong>for</strong> prevention of stroke andsystemic embolism (SEE) associated with atrial fibrillation.Safety and efficacy of rivaroxaban have not been established in pregnant women. Studies in animals haveshown reproductive toxicity. Due to the potential reproductive toxicity, the intrinsic risk of bleeding andevidence that rivaroxaban crosses the placenta, rivaroxaban is contraindicated during pregnancy.When clinically relevant bleeding occurs, treatment should be interrupted. Bleeding can occur at any siteduring therapy with rivaroxaban. An unexplained fall in haemoglobin and/or haematocrit or blood pressureshould lead to a search <strong>for</strong> a bleeding siteFor subjects with gastritis, oesophagitis, or gastroesophageal reflux, the lower dose of 15mg may beconsidered due to the elevated risk of major gastro-intestinal bleeding. As with warfarin, co-administration ofaspirin, clopidogrel and NSAID increases risk of bleeding.The administration of a PPI can be considered to help prevent GI bleeding.If a dose is missed the patient should take rivaroxaban immediately and then continue the following day withonce daily intake as be<strong>for</strong>e. No double dose should be taken to make up <strong>for</strong> a missed dose.Caution - Concomitant administration of strong CYP3A4 and P-gp inhibitors (such as amiodarone, verapamil,quinidine, ketoconazole and clarithromycin) is expected to result in increased rivaroxaban plasmaconcentrations and potential increase in bleeding riskVersion 8Lisa Rogan on behalf of Lancashire and <strong>Cumbria</strong> Health Economy New Medicines and Treatments Group – September 2012Updated by Lesley Davey of Lancashire Medicines Management Group to include apixaban – May 2013 – Review Date - May 2015This guidance does not override the individual responsibility of health professionals to make decisions in exercising their clinical judgement in the circumstances of theindividual patient, in consultation with the patient and/or guardian or carer. For full prescribing in<strong>for</strong>mation please refer to the BNF and SPC ensuring correct SPCaccording to dose is consulted.
FURTHER PRESCRIBER GUIDANCE FOR APIXABAN (Eliquis )Interaction with other medicinal products affecting haemostasisDue to an increased bleeding risk, concomitant treatment with any other anticoagulants iscontraindicated.Agents associated with serious bleeding are not recommended concomitantly with apixaban, suchas: thrombolytic agents, GPIIb/IIIa receptor antagonists, thienopyridines (e.g., clopidogrel),dipyridamole, dextran and sulfinpyrazone.Care is to be taken if patients are treated concomitantly with non-steroidal anti-inflammatory drugs (NSAIDs),including acetylsalicylic acid, especially in elderly patients because of a potentially higher bleeding risk.Following surgery, other platelet aggregation inhibitors are not recommended concomitantly with apixaban.In patients with atrial fibrillation and conditions that warrant mono or dual antiplatelet therapy, a carefulassessment of the potential benefits against the potential risks should be made be<strong>for</strong>e combining thistherapy with apixaban.In a clinical trial of patients with atrial fibrillation, concomitant use of acetylsalicylic acid increased the majorbleeding risk on apixaban almost 2-fold.In a clinical trial of high-risk post acute coronary syndrome patients, who received acetylsalicylic acid or thecombination of acetylsalicylic acid and clopidogrel, apixaban related major bleeding was reported to be twoand a half – fold higher than placebo.The use of Apixaban is not recommended in patients receiving concomitant systemic treatment with stronginhibitors of both CYP3A4 and P-gp, such as as azole-antimycotics (e.g., ketoconazole, itraconazole,voriconazole and posaconazole) and HIV protease inhibitors (e.g., ritonavir)No dose adjustment <strong>for</strong> apixaban is required when co-administered with less potent inhibitors of CYP3A4and/or P-gp such as diltiazem, naproxen, amiodarone, verapamil and quinidine.Co-administration of apixaban with rifampicin, a strong inducer of both CYP3A4 and P-gp, led to anapproximate 54% and 42% decrease in mean apixaban AUC and C max , respectively. The concomitant use ofapixaban with other strong CYP3A4 and P-gp inducers (e.g., phenytoin, carbamazepine, phenobarbital or St.John's Wort) may also lead to reduced apixaban plasma concentrations. No dose adjustment <strong>for</strong> apixaban isrequired during concomitant therapy with such agents, however strong inducers of both CYP3A4 and P-gpshould be co-administered with caution.Other concomitant therapiesNo clinically significant pharmacokinetic or pharmacodynamic interactions were observed when apixabanwas co-administered with atenolol or famotidine.Effect of apixaban on other medicinal productsApixaban is not expected to alter the clearance of co-administered drugs metabolised by CYP1A2, CYP2A6,CYP2B6, CYP2C8, CYP2C9, CYP2D6 or CYP3A4 (IC50 > 45 μM) CYP2C19 (IC50 > 20 μM)Apixaban is not a significant inhibitor of P-gp.In studies conducted in healthy subjects, as described below, apixaban did not meaningfully alter thepharmacokinetics of digoxin, naproxen, or atenolol.Spinal/epidural anaesthesia or punctureExperience with neuraxial blockade is limited and extreme caution is there<strong>for</strong>e recommended when usingapixaban. Prior to neuraxial intervention the physician should consider the potential benefit versus the risk inanticoagulated patients or in patients to be anticoagulated <strong>for</strong> thromboprophylaxis.To reduce the risk of thromboembolic complications, indwelling epidural or intrathecal catheters must be removedat least 5 hours prior to the first dose of apixaban. The risk of developing an epidural or spinal haematoma whichcan result in long-term or permanent paralysis may be increased by the post-operative use of indwelling epiduralcatheters or the concomitant use of medicinal products affecting haemostasis. Indwelling epidural or intrathecalcatheters must be removed at least 5 hours prior to the first dose of apixaban. The risk may also be increased bytraumatic or repeated epidural or spinal puncture. Patients are to be frequently monitored <strong>for</strong> signs and symptomsof neurological impairment. If neurological compromise is noted, urgent diagnosis and treatment isnecessary.There is no clinical experience with the use of apixaban with indwelling intrathecal or epidural catheters.In case there is such need, a time interval of 20-30 hours (i.e., 2 x half-life) between the last dose of apixaban andcatheter withdrawal should elapse, and at least one dose should be omitted be<strong>for</strong>e catheter withdrawal. The nextdose of apixaban may be given at least 5 hours after catheter removal.
There is no antidote to apixaban.Activated charcoal may be useful in the management of apixaban overdose or accidental ingestion.Overdose of apixaban may result in a higher risk of bleeding. If bleeding occurs, discontinue treatment andinvestigate the source of bleeding. The initiation of appropriate treatment, e.g., surgical haemostasis or thetransfusion of fresh frozen plasma should be considered.If life-threatening bleeding cannot be controlled by the above measures, administration of recombinant factor VIIamay be considered. However, there is currently no experience with the use of recombinant factor VIIa in individualsreceiving apixaban. Re-dosing of recombinant factor VIIa could be considered and titrated depending onimprovement of bleeding.As with other anticoagulants, patients taking apixaban are to be carefully observed <strong>for</strong> signs of bleeding. It isrecommended to be used with caution in conditions with increased risk of haemorrhage. Apixaban administrationshould be discontinued if severe haemorrhage occurs.Although treatment with apixaban does not require routine monitoring of exposure, anti-FXa assay may be useful inexceptional situations where knowledge of apixaban exposure may help to in<strong>for</strong>m clinical decisions, e.g. overdoseand emergency surgery.Prior to initiating apixaban, liver function testing should be per<strong>for</strong>med.Apixaban is contraindicated in patients with hepatic disease associated with coagulopathy and clinically relevantbleeding risk.It is not recommended in patients with severe hepatic impairment.It should be used with caution in patients with mild or moderate hepatic impairment (Child Pugh A or B).It should be used with caution in patients with elevated liver enzymes ALT/AST > 2 x ULN or total bilirubin ≥ 1.5 xULN as they were excluded from clinical trials.If a dose is missed, the patient should take apixaban immediately and then continue with twice daily intake as be<strong>for</strong>e.There are no data from the use of apixaban in pregnant women. Animal studies do not indicate direct or indirectharmful effects with respect to reproductive toxicity. Apixaban is not recommended during pregnancy.Breast-feedingIt is unknown whether apixaban or its metabolites are excreted in human milk. Available data in animals have shownexcretion of apixaban in milk. In rat milk, a high milk to maternal plasma ratio (C max about 8, AUC about 30) wasfound, possibly due to active transport into the milk. A risk to newborns and infants cannot be excluded.A decision must be made to either discontinue breast-feeding or to discontinue/abstain from apixaban therapy.FertilityStudies in animals dosed with apixaban have shown no effect on fertility.References:NICE TAG - 256SPC Xarelto® accessed via http://www.medicines.org.uk/emc/medicine/25586/SPC/Xarelto+20+mg+film-coated+tablets/<strong>NHS</strong> Fife Area DTC – March 2012NICE TAG 249SPC Pradaxa® accessed via http://www.medicines.org.uk/EMC/medicine/24839/SPC/Pradaxa+150+mg+hard+capsules/http://www.medicines.org.uk/EMC/medicine/20760/SPC/Pradaxa+110+mg+hard+capsules/NICE TAG 275SPC Eliquis® accessed viahttp://www.medicines.org.uk/emc/medicine/27220/SPC/Eliquis+5+mg+film-coated+tablets/http://www.medicines.org.uk/emc/medicine/24988/SPC/Eliquis+2.5+mg+film-coated+tablets/Acknowledgement: Somerset PCTAuthors: Lisa Rogan – <strong>NHS</strong> East Lancashire, Lesley Davey – <strong>NHS</strong> Lancashire CSUVersion 8Lisa Rogan on behalf of Lancashire and <strong>Cumbria</strong> Health Economy New Medicines and Treatments Group – September 2012Updated by Lesley Davey of Lancashire Medicines Management Group to include apixaban – May 2013 – Review Date - May 2015This guidance does not override the individual responsibility of health professionals to make decisions in exercising their clinical judgement in the circumstances of theindividual patient, in consultation with the patient and/or guardian or carer. For full prescribing in<strong>for</strong>mation please refer to the BNF and SPC ensuring correct SPCaccording to dose is consulted.