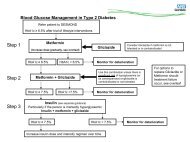
Prescribing Guidance for NOACs - NHS Cumbria
Prescribing Guidance for NOACs - NHS Cumbria
Prescribing Guidance for NOACs - NHS Cumbria
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
FURTHER PRESCRIBER GUIDANCE FOR RIVAROXABAN (Xarelto )Interactions with other medicines and other relevant interactionsThe use of rivaroxaban is not recommended in patients receiving concomitant systemic treatment with azoleantimycoticssuch as ketoconazole, itraconazole, voriconazole and posaconazole or HIV protease inhibitors.These active substances are strong inhibitors of both CYP3A4 and P-gp.Interactions with Clarithromycin, erythromycin and fluconazole are not considered clinically significant (SPC).Given the limited clinical data available with dronedarone, co-administration with rivaroxaban should beavoided.Care is to be taken if patients are treated concomitantly with NSAIDs (including acetylsalicylic acid) andplatelet aggregation inhibitors because these medicinal products typically increase the bleeding risk..No pharmacokinetic interaction was observed between warfarin and rivaroxaban.The concomitant use of rivaroxaban with other strong CYP3A4 inducers (e.g. phenytoin, carbamazepine,phenobarbital or St. John's Wort) may lead to reduced rivaroxaban plasma concentrations. Strong CYP3A4inducers should be co-administered with caution.In case of intolerability to rivaroxaban, patients should be instructed to immediately consult their treatingphysician in order to be switched to alternate acceptable treatment options <strong>for</strong> prevention of stroke andsystemic embolism (SEE) associated with atrial fibrillation.Safety and efficacy of rivaroxaban have not been established in pregnant women. Studies in animals haveshown reproductive toxicity. Due to the potential reproductive toxicity, the intrinsic risk of bleeding andevidence that rivaroxaban crosses the placenta, rivaroxaban is contraindicated during pregnancy.When clinically relevant bleeding occurs, treatment should be interrupted. Bleeding can occur at any siteduring therapy with rivaroxaban. An unexplained fall in haemoglobin and/or haematocrit or blood pressureshould lead to a search <strong>for</strong> a bleeding siteFor subjects with gastritis, oesophagitis, or gastroesophageal reflux, the lower dose of 15mg may beconsidered due to the elevated risk of major gastro-intestinal bleeding. As with warfarin, co-administration ofaspirin, clopidogrel and NSAID increases risk of bleeding.The administration of a PPI can be considered to help prevent GI bleeding.If a dose is missed the patient should take rivaroxaban immediately and then continue the following day withonce daily intake as be<strong>for</strong>e. No double dose should be taken to make up <strong>for</strong> a missed dose.Caution - Concomitant administration of strong CYP3A4 and P-gp inhibitors (such as amiodarone, verapamil,quinidine, ketoconazole and clarithromycin) is expected to result in increased rivaroxaban plasmaconcentrations and potential increase in bleeding riskVersion 8Lisa Rogan on behalf of Lancashire and <strong>Cumbria</strong> Health Economy New Medicines and Treatments Group – September 2012Updated by Lesley Davey of Lancashire Medicines Management Group to include apixaban – May 2013 – Review Date - May 2015This guidance does not override the individual responsibility of health professionals to make decisions in exercising their clinical judgement in the circumstances of theindividual patient, in consultation with the patient and/or guardian or carer. For full prescribing in<strong>for</strong>mation please refer to the BNF and SPC ensuring correct SPCaccording to dose is consulted.