Nycomed Annual Report 2008 - Takeda Pharmaceuticals ...
Nycomed Annual Report 2008 - Takeda Pharmaceuticals ...
Nycomed Annual Report 2008 - Takeda Pharmaceuticals ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
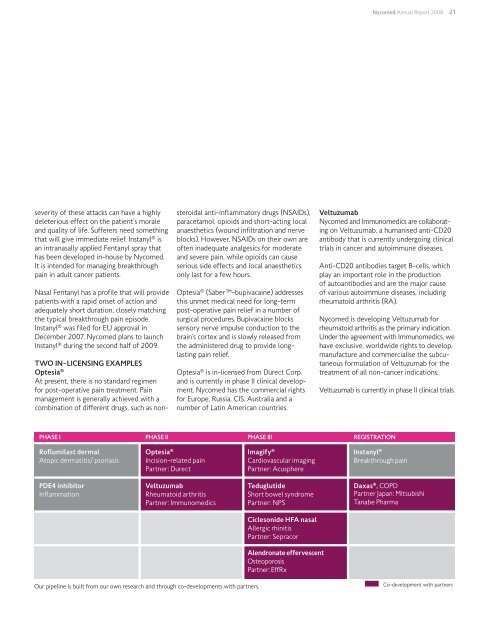
<strong>Nycomed</strong> <strong>Annual</strong> <strong>Report</strong> <strong>2008</strong> 21severity of these attacks can have a highlydeleterious effect on the patient’s moraleand quality of life. Sufferers need somethingthat will give immediate relief. Instanyl ® isan intranasally applied Fentanyl spray thathas been developed in-house by <strong>Nycomed</strong>.It is intended for managing breakthroughpain in adult cancer patients.Nasal Fentanyl has a profile that will providepatients with a rapid onset of action andadequately short duration, closely matchingthe typical breakthrough pain episode.Instanyl ® was filed for EU approval inDecember 2007. <strong>Nycomed</strong> plans to launchInstanyl ® during the second half of 2009.Two in-licensing examplesOptesia ®At present, there is no standard regimenfor post-operative pain treatment. Painmanagement is generally achieved with acombination of different drugs, such as nonsteroidalanti-inflammatory drugs (NSAIDs),paracetamol, opioids and short-acting localanaesthetics (wound infiltration and nerveblocks). However, NSAIDs on their own areoften inadequate analgesics for moderateand severe pain, while opioids can causeserious side effects and local anaestheticsonly last for a few hours.Optesia ® (Saber-bupivacaine) addressesthis unmet medical need for long-termpost-operative pain relief in a number ofsurgical procedures. Bupivacaine blockssensory nerve impulse conduction to thebrain’s cortex and is slowly released fromthe administered drug to provide longlastingpain relief.Optesia ® is in-licensed from Durect Corp.and is currently in phase II clinical development.<strong>Nycomed</strong> has the commercial rightsfor Europe, Russia, CIS, Australia and anumber of Latin American countries.Veltuzumab<strong>Nycomed</strong> and Immunomedics are collaboratingon Veltuzumab, a humanised anti-CD20antibody that is currently undergoing clinicaltrials in cancer and autoimmune diseases.Anti-CD20 antibodies target B-cells, whichplay an important role in the productionof autoantibodies and are the major causeof various autoimmune diseases, includingrheumatoid arthritis (RA).<strong>Nycomed</strong> is developing Veltuzumab forrheumatoid arthritis as the primary indication.Under the agreement with Immunomedics, wehave exclusive, worldwide rights to develop,manufacture and commercialise the subcutaneousformulation of Veltuzumab for thetreatment of all non-cancer indications.Veltuzumab is currently in phase II clinical trials.Clinical development pipeline features promising projectsPhase I Phase II Phase III RegistrationRoflumilast dermalAtopic dermatitis/ psoriasisOptesia ®Incision-related painPartner: DurectImagify ®Cardiovascular imagingPartner: AcusphereInstanyl ®Breakthrough painPDE4 inhibitorInflammationVeltuzumabRheumatoid arthritisPartner: ImmunomedicsTeduglutideShort bowel syndromePartner: NPSDaxas ® , COPDPartner Japan: MitsubishiTanabe PharmaCiclesonide HFA nasalAllergic rhinitisPartner: SepracorAlendronate effervescentOsteoporosisPartner: EffRxOur pipeline is built from our own research and through co-developments with partners. Co-development with partners