Laser-Induced Photocatalysis and its Applications in Petrochemicals ...
Laser-Induced Photocatalysis and its Applications in Petrochemicals ...
Laser-Induced Photocatalysis and its Applications in Petrochemicals ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
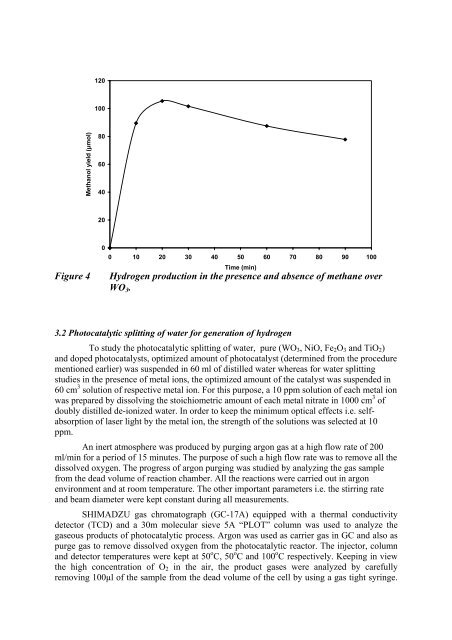
120100Methanol yield (µmol)80604020Figure 400 10 20 30 40 50 60 70 80 90 100Time (m<strong>in</strong>)Hydrogen production <strong>in</strong> the presence <strong>and</strong> absence of methane overWO 3 .3.2 Photocatalytic splitt<strong>in</strong>g of water for generation of hydrogenTo study the photocatalytic splitt<strong>in</strong>g of water, pure (WO 3 , NiO, Fe 2 O 3 <strong>and</strong> TiO 2 )<strong>and</strong> doped photocatalysts, optimized amount of photocatalyst (determ<strong>in</strong>ed from the procedurementioned earlier) was suspended <strong>in</strong> 60 ml of distilled water whereas for water splitt<strong>in</strong>gstudies <strong>in</strong> the presence of metal ions, the optimized amount of the catalyst was suspended <strong>in</strong>60 cm 3 solution of respective metal ion. For this purpose, a 10 ppm solution of each metal ionwas prepared by dissolv<strong>in</strong>g the stoichiometric amount of each metal nitrate <strong>in</strong> 1000 cm 3 ofdoubly distilled de-ionized water. In order to keep the m<strong>in</strong>imum optical effects i.e. selfabsorptionof laser light by the metal ion, the strength of the solutions was selected at 10ppm.An <strong>in</strong>ert atmosphere was produced by purg<strong>in</strong>g argon gas at a high flow rate of 200ml/m<strong>in</strong> for a period of 15 m<strong>in</strong>utes. The purpose of such a high flow rate was to remove all thedissolved oxygen. The progress of argon purg<strong>in</strong>g was studied by analyz<strong>in</strong>g the gas samplefrom the dead volume of reaction chamber. All the reactions were carried out <strong>in</strong> argonenvironment <strong>and</strong> at room temperature. The other important parameters i.e. the stirr<strong>in</strong>g rate<strong>and</strong> beam diameter were kept constant dur<strong>in</strong>g all measurements.SHIMADZU gas chromatograph (GC-17A) equipped with a thermal conductivitydetector (TCD) <strong>and</strong> a 30m molecular sieve 5A “PLOT” column was used to analyze thegaseous products of photocatalytic process. Argon was used as carrier gas <strong>in</strong> GC <strong>and</strong> also aspurge gas to remove dissolved oxygen from the photocatalytic reactor. The <strong>in</strong>jector, column<strong>and</strong> detector temperatures were kept at 50 o C, 50 o C <strong>and</strong> 100 o C respectively. Keep<strong>in</strong>g <strong>in</strong> viewthe high concentration of O 2 <strong>in</strong> the air, the product gases were analyzed by carefullyremov<strong>in</strong>g 100µl of the sample from the dead volume of the cell by us<strong>in</strong>g a gas tight syr<strong>in</strong>ge.