Reduction of Chromium Oxide in Stainless Steel Slags - Pyro.co.za
Reduction of Chromium Oxide in Stainless Steel Slags - Pyro.co.za
Reduction of Chromium Oxide in Stainless Steel Slags - Pyro.co.za
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
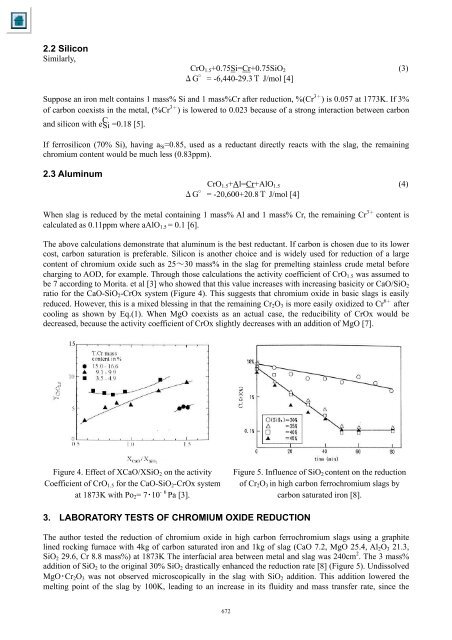
2.2 Sili<strong>co</strong>nSimilarly,CrO 1.5 +0.75Si=Cr+0.75SiO 2 (3)ΔG°= -6,440-29.3Τ J/mol [4]Suppose an iron melt <strong>co</strong>nta<strong>in</strong>s 1 mass% Si and 1 mass%Cr after reduction, %(Cr 3+ ) is 0.057 at 1773K. If 3%<strong>of</strong> carbon <strong>co</strong>exists <strong>in</strong> the metal, (%Cr 3+ ) is lowered to 0.023 because <strong>of</strong> a strong <strong>in</strong>teraction between carbonand sili<strong>co</strong>n with e C Si =0.18 [5].If ferrosili<strong>co</strong>n (70% Si), hav<strong>in</strong>g a Si =0.85, used as a reductant directly reacts with the slag, the rema<strong>in</strong><strong>in</strong>gchromium <strong>co</strong>ntent would be much less (0.83ppm).2.3 Alum<strong>in</strong>umCrO 1.5 +Al=Cr+AlO 1.5 (4)ΔG°= -20,600+20.8Τ J/mol [4]When slag is reduced by the metal <strong>co</strong>nta<strong>in</strong><strong>in</strong>g 1 mass% Al and 1 mass% Cr, the rema<strong>in</strong><strong>in</strong>g Cr 3+ <strong>co</strong>ntent iscalculated as 0.11ppm where aAlO 1.5 = 0.1 [6].The above calculations demonstrate that alum<strong>in</strong>um is the best reductant. If carbon is chosen due to its lower<strong>co</strong>st, carbon saturation is preferable. Sili<strong>co</strong>n is another choice and is widely used for reduction <strong>of</strong> a large<strong>co</strong>ntent <strong>of</strong> chromium oxide such as 25~30 mass% <strong>in</strong> the slag for premelt<strong>in</strong>g sta<strong>in</strong>less crude metal beforecharg<strong>in</strong>g to AOD, for example. Through those calculations the activity <strong>co</strong>efficient <strong>of</strong> CrO 1.5 was assumed tobe 7 ac<strong>co</strong>rd<strong>in</strong>g to Morita. et al [3] who showed that this value <strong>in</strong>creases with <strong>in</strong>creas<strong>in</strong>g basicity or CaO/SiO 2ratio for the CaO-SiO 2 -CrOx system (Figure 4). This suggests that chromium oxide <strong>in</strong> basic slags is easilyreduced. However, this is a mixed bless<strong>in</strong>g <strong>in</strong> that the rema<strong>in</strong><strong>in</strong>g Cr 2 O 3 is more easily oxidized to Cr 6+ after<strong>co</strong>ol<strong>in</strong>g as shown by Eq.(1). When MgO <strong>co</strong>exists as an actual case, the reducibility <strong>of</strong> CrOx would bedecreased, because the activity <strong>co</strong>efficient <strong>of</strong> CrOx slightly decreases with an addition <strong>of</strong> MgO [7].Figure 4. Effect <strong>of</strong> XCaO/XSiO 2 on the activityCoefficient <strong>of</strong> CrO 1.5 for the CaO-SiO 2 -CrOx systemat 1873K with Po 2 = 7・10 - 6 Pa [3].Figure 5. Influence <strong>of</strong> SiO 2 <strong>co</strong>ntent on the reduction<strong>of</strong> Cr 2 O 3 <strong>in</strong> high carbon ferrochromium slags bycarbon saturated iron [8].3. LABORATORY TESTS OF CHROMIUM OXIDE REDUCTIONThe author tested the reduction <strong>of</strong> chromium oxide <strong>in</strong> high carbon ferrochromium slags us<strong>in</strong>g a graphitel<strong>in</strong>ed rock<strong>in</strong>g furnace with 4kg <strong>of</strong> carbon saturated iron and 1kg <strong>of</strong> slag (CaO 7.2, MgO 25.4, Al 2 O 3 21.3,SiO 2 29.6, Cr 8.8 mass%) at 1873K The <strong>in</strong>terfacial area between metal and slag was 240cm 2 . The 3 mass%addition <strong>of</strong> SiO 2 to the orig<strong>in</strong>al 30% SiO 2 drastically enhanced the reduction rate [8] (Figure 5). UndissolvedMgO・Cr 2 O 3 was not observed micros<strong>co</strong>pically <strong>in</strong> the slag with SiO 2 addition. This addition lowered themelt<strong>in</strong>g po<strong>in</strong>t <strong>of</strong> the slag by 100K, lead<strong>in</strong>g to an <strong>in</strong>crease <strong>in</strong> its fluidity and mass transfer rate, s<strong>in</strong>ce the