189 Coronaviruses and Toroviruses, Including Severe Acute ...
189 Coronaviruses and Toroviruses, Including Severe Acute ...
189 Coronaviruses and Toroviruses, Including Severe Acute ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
CHAPTER <strong>189</strong> <strong>Coronaviruses</strong> <strong>and</strong> <strong>Toroviruses</strong>, <strong>Including</strong> <strong>Severe</strong> <strong>Acute</strong> Respiratory Syndrome (SARS) 237912. Johnson, E. D., Roimet, E., Gitau, L. G., et al.: Marburg virus disease: Anenvironmental health threat in Kenya. In Kinoti, S. H., Waiyoki, P. G.,<strong>and</strong> Were, B. D. (eds.): Proceedings of the 11th Annual Medical ScientificConference. Nairobi, Kenya, African Medical Research Foundation, 1990.13. Johnson, K. M., Webb, P. A., Lange, J., et al.: Isolation <strong>and</strong> partial characterizationof a new virus causing acute haemorrhagic fever in Zaire.Lancet 1:569–571, 1977.14. Kissling, R. E., Robinson, R. Q., Murphy, F. A., et al.: Agent of diseasecontracted from green monkeys. Science 160:888–890, 1968.15. Ksiazek, T. G., Rollin, P. E., Jahrling, P. B., et al.: Enzyme immunosorbentassay for Ebola virus antigens in tissues of infected primates. J. Clin.Microbiol. 30:947–950, 1992.16. Kudoyarova-Zubavichene, N. M., Sergeyev, N. N., Chepurnov, A. A.,et al.: Preparation <strong>and</strong> use of hyperimmune serum for prophylaxis <strong>and</strong>therapy of Ebola virus infections. J. Infect. Dis. 179(Suppl. 1):218–223,1999.17. Le Guenno, B., Formentry, P., Wyers, M., et al.: Isolation <strong>and</strong> partialcharacterization of a new strain of Ebola virus. Lancet 345:1271–1274.18. Martini, G. A., Knauff, H. G., Schmidt, H. A., et al.: A previouslyunknown infectious disease contracted from monkeys: Marburg virus disease.Dtsch. Med. Wochenschr. 93:559–571, 1968.19. Martini, G. A., <strong>and</strong> Siegert, R. (ed.): Marburg Virus Disease. New York,Springer-Verlag, 1971.20. Mikhailov, V. V., Borisevich, I. V., Chernikov, N. K., et al.: The evaluationin hamadryas baboons of the possibility for the specific prevention ofEbola fever. Vopr. Virusol. 39:82–84, 1994.21. Monath, T. P.: Ecology of Marburg <strong>and</strong> Ebola viruses: Speculations <strong>and</strong>directions for future research. J. Infect. Dis. 179(Suppl. 1):127–138, 1999.22. Mupapa, K., Massamba, M., Kibadi, K., et al.: Treatment of Ebola hemorrhagicfever with blood transfusions from convalescent patients. J.Infect. Dis. 179(Suppl.):18–23, 1999.23. Netesov, S. V., Feldmann, H., Jahrling, P. B., et al.: Family Filoviridae.Reoviridae. In Van Regenmortel, M. H. V., Fauquet, C. M., Bishop, D. H.L., et al. (eds.): Virus Taxonomy. Seventh Report of the InternationalCommittee for the Taxonomy of Viruses. New York, Academic, 2000, pp.539–548.24. Outbreak of Ebola hemorrhagic fever—Ug<strong>and</strong>a, August 2000–January2001. M. M. W. R. Morb. Mortal. Wkly. Rep.50:73–77, 2001.25. Peters, C. J., Buchmeier, M., Rollin, P. E., <strong>and</strong> Ksiazek, T. G.: Filoviridae:Marburg <strong>and</strong> Ebola viruses. In Fields, B. N., Knipe, D. M., Howley, P. M.,et al. (eds.): Fields Virology. 3rd ed. Philadelphia, Lippincott-Raven, 1996,pp. 1161–1176.26. Peters, C. J., Sanchez, A., Rollin, P. E., et al.: Filoviridae: Marburg <strong>and</strong>Ebola viruses. In Belshe, B. (ed.): Textbook of Human Virology. 4th ed.St. Louis, Mosby–Year Book, 1996, p. 699.27. Reiter, P., Turell, M., Coleman, R., et al.: Field investigations of an outbreakof Ebola hemorrhagic fever, Kikwit, Democratic Republic of theCongo, 1995: Arthropod studies. J. Infect. Dis. 179(Suppl. 1):148–154,1999.28. Rollin, P. E., Williams, R. J., Bressler, D. S., et al.: Ebola (subtype Reston)virus among quarantined nonhuman primates recently imported fromthe Philippines to the United States. J. Infect. Dis. 179(Suppl. 1):108–114,1999.29. Smith, C. E. G., Simpson, D. I. H., Bowen, E. T. W., et al.: Fatal hum<strong>and</strong>isease from vervet monkeys. Lancet 2:1119–1121, 1967.30. Smith, D. H., Johnson, B. K., Isaacson, M., et al.: Marburg virus diseasein Kenya. Lancet 1:816–820, 1982.31. Stille, W., Boehle, E., Heim, E., et al.: An infectious disease transmittedby Cercopthecus aethiops. Dtsch. Med. Wochenschr. 93:572–582, 1968.32. Sullivan, N. J., Sanchez, A., Rollin, P. E., et al.: Development of a preventivevaccine for Ebola virus infection in primates. Nature408:605–609, 2000.33. Swanepoel, R., Leman, P. A., Burt, F. J., et al.: Experimental inoculationof plants <strong>and</strong> animals with Ebola virus. Emerg. Infect. Dis. 2:321–325,1996.34. Update: Management of patients with suspected viral hemorrhagic fever.M. M. W. R. Morb. Mortal. Wkly. Rep. 44:475–479, 1995.35. Update: Outbreak of Ebola virus hemorrhagic fever—Zaire, 1995. M. M.W. R. Morb. Mortal. Wkly. Rep. 44:399, 1995.36. Villinger, F., Rollin, P. E., Brar, S. S. et al.: Markedly elevated levels ofinterferon (IFN)-α, IFN-γ, interleukin (IL)-2, IL-10, <strong>and</strong> tumor necrosisfactor-α associated with fatal Ebola virus infection. J. Infect. Dis.179(Suppl. 1):188–191, 1999.37. Viral haemorrhagic fever in imported monkeys. Wkly. Epidemiol. Rec.67:142–143, 1992.38. Viral haemorrhagic fever/Marburg, Democratic Republic of the Congo.Wkly. Epidemiol. Rec. 74:157–158, 1999.39. Viral haemorrhagic fever surveillance. Wkly. Epidemiol. Rec. 44:2, 1979.40. Zaki, S. R., <strong>and</strong> Goldsmith, C. S.: Pathologic features of filovirus infectionsin humans. Curr. Top. Microbiol. Immunol. 235:97–116, 1999.41. Zaki, S. R., Shieh, W.-J., Greer, P. W., et al.: A novel immunohistochemicalassay for the detection of Ebola virus in skin: Implications for diagnosis,spread, <strong>and</strong> surveillance of Ebola hemorrhagic fever. J. Infect. Dis.179(Suppl. 1):36–47, 1999.SUBSECTION 10CORONAVIRIDAECHAPTER<strong>189</strong> <strong>Coronaviruses</strong> <strong>and</strong> <strong>Toroviruses</strong>, <strong>Including</strong><strong>Severe</strong> <strong>Acute</strong> Respiratory Syndrome (SARS)CHAPTER<strong>189</strong>A<strong>Coronaviruses</strong> <strong>and</strong> <strong>Toroviruses</strong>KENNETH McINTOSHThe family Coronaviridae is composed of two genera,Coronavirus <strong>and</strong> Torovirus. The two genera are related in thatthey appear quite similar on electron microscopy <strong>and</strong> theyshare similar strategies of replication (along with other membersof the order Nidovirales, the arteriviruses). They differ,however, in the size of their RNA genome <strong>and</strong> structuralproteins, as well as in the morphology of their nucleocapsids.<strong>Coronaviruses</strong> are primarily respiratory pathogens inhumans, although they cause a wide variety of importantdiseases in animals, including infectious bronchitis <strong>and</strong>nephrosis in chickens; gastroenteritis <strong>and</strong> encephalitisin young piglets; enteritis in turkeys, dogs, <strong>and</strong> calves;hepatitis <strong>and</strong> encephalitis in mice; pneumonitis <strong>and</strong> sialodacryoadenitisin rats; <strong>and</strong> infectious peritonitis in cats. 48<strong>Toroviruses</strong> are, at least as presently known, exclusivelyenteric pathogens, both in animals <strong>and</strong> humans.Since the first report of human coronavirus (HCoV) isolationin 1965, 104 the HCoV group of RNA viruses has beenconfirmed as a frequent cause of the common cold in children<strong>and</strong> adults. They also have been implicated as possible
CHAPTER <strong>189</strong> <strong>Coronaviruses</strong> <strong>and</strong> <strong>Toroviruses</strong>, <strong>Including</strong> <strong>Severe</strong> <strong>Acute</strong> Respiratory Syndrome (SARS) 2381ABFIGURE <strong>189</strong>–1 ■ Negatively stained virus particles in stool samples representing a typical intestinal coronavirus (A) <strong>and</strong> torovirus (B).The bar represents 100 nm. The particles both show the typical petal-shaped projections, but those of the coronaviruses are more finelyformed <strong>and</strong> distinct than those of the toroviruses. (Photomicrograph kindly provided by Dr. Martin Petric, Department of LaboratoryMedicine, Hospital for Sick Children, Toronto, Canada.)<strong>Toroviruses</strong> differ from enteric coronaviruses in beingsomewhat smaller <strong>and</strong> more pleomorphic <strong>and</strong> in havingsomewhat less distinct surface projections. 22<strong>Toroviruses</strong> contain S glycoproteins on their surface, butno significant sequence homology seems to exist betweenthem <strong>and</strong> the S proteins of coronaviruses. 98 A second surfaceprotein with HE activity <strong>and</strong> sequence homology to the HEproteins of both influenza C virus <strong>and</strong> mouse hepatitis virushas been found on Breda virus, the bovine torovirus, but noton the equine Berne virus. 19 Whether this molecule exists onhuman toroviruses is not known, although humantoroviruses do hemagglutinate rabbit erythrocytes. 22<strong>Toroviruses</strong> contain membranes <strong>and</strong> nucleoproteins similarto those of coronaviruses but with no sequence homology.On the other h<strong>and</strong>, the replicase contains sequence similarityto that of coronaviruses, <strong>and</strong> their replication strategyappears to be very similar. 98 Greater than 90 percent identityin the 3′ end of the genome exists between human <strong>and</strong>22, 98animal toroviruses.The replication strategy of toroviruses probably is verysimilar to that of coronaviruses in view of the similar organizationof the genome <strong>and</strong> the sequence similarity of theirreplicase genes.EpidemiologyBecause of the difficulty in isolating respiratory HCoV, mostepidemiologic data are derived from serologic surveillance,usually complement fixation, hemagglutination inhibition,neutralization, or enzyme-linked immunosorbent assay(ELISA), with HCoV-229E or HCoV-OC43 viruses used as13, 46, 54antigens. Complement-fixation antibody titersfrequently are transient, whereas hemagglutination inhibition,neutralization, <strong>and</strong> ELISA often reflect infectionmonths or years in the past. Because enteric HCoVs rarelyhave been propagated in culture, epidemiologic study ofthese agents has been hindered by lack of antigens for serology.Likewise, the epidemiology of human toroviruses hasnot been well delineated. What information is availableabout the epidemiology of both enteric coronaviruses<strong>and</strong> toroviruses is outlined in the later section on clinicalmanifestations.GEOGRAPHIC PREVALENCECoronavirus infections occur worldwide. Seroprevalencestudies of HCoV-229E <strong>and</strong> HCoV-OC43 infection have been9, 17,conducted in the United States, Europe, Brazil, <strong>and</strong> Iraq.32, 33, 41, 42, 67 With ELISA, antibody prevalence in adults fromall areas where they have been examined approaches 90 to100 percent.SEASONAL INCIDENCE AND ANNUAL RECYCLINGPATTERNAlthough HCoV infection may occur at any time of the year,most are seen in midwinter to early spring. Individual serotypestypically predominate in one year, <strong>and</strong> this patternthen is followed by one or more years of low activity. In
2382 SECTION XVII Viral Infectionshealthy children in Atlanta, Georgia, prospectively examinedfor HCoV-229E <strong>and</strong> HCoV-OC43 infection from 1960through 1968 (Fig. <strong>189</strong>–2), the incidence of autumn infectionsapproached that of spring infections, <strong>and</strong> a few infectionsusually occurred in the summer. In addition, althougha very considerable HCoV-229E outbreak occurred in 1961to 1962, followed by 2 years with a low incidence, in thesubsequent 4 years the incidence was quite similar from41, 42year to year.Whatever the season, a single HCoV serotype can cause,at least locally, a high incidence of infection, such as thatcaused by HCoV-229E in Atlanta children in 1961 to 1962(see Fig. <strong>189</strong>–2). HCoV-229E also was associated with avery significant outbreak in medical students at theUniversity of Chicago in 1966 <strong>and</strong> 1967. 30 Very substantialproportions of the population can be infected during theseintervals. Among the Chicago medical students, 66 (35%)of 191 were infected. In Tecumseh, Michigan, a largeHCoV-229E outbreak that occurred between January <strong>and</strong>April 1967 affected 68 percent of 38 families <strong>and</strong> 34 percentof 159 individuals tested. Sharp outbreaks also can occur incertain hospitalized infants: at National Jewish Hospital inDenver, 16 of 20 hospitalized asthmatic infants wereinfected with HCoV-OC43 in December 1968. 65RATIO OF CLINICAL TO SUBCLINICAL ILLNESSIn healthy children <strong>and</strong> adults, HCoVs often are shedasymptomatically. In the 8.5-year surveillance of healthyolder children in Atlanta (see Fig. <strong>189</strong>–2), only 38 <strong>and</strong> 47percent of HCoV-229E <strong>and</strong> HCoV-OC43 seroconversions,respectively, were associated with respiratory illness. In agroup of infants <strong>and</strong> young children in metropolitan Washington,D.C., tested for HCoV-OC43 seroconversion, at least50 percent of the infections were subclinical. 67 Susceptibilityto HCoV-induced disease may be much greater in certainpopulations. In the Denver children hospitalized with atopicasthma, 19 were infected with either HCoV-229E or HCoV-OC43 <strong>and</strong> 17 were symptomatic. 65AGE SPECIFICITY OF INFECTIONAntibody to both HCoV-229E <strong>and</strong> HCoV-OC43 appears inearly childhood, <strong>and</strong> the prevalence increases rapidly withage. On the other h<strong>and</strong>, asymptomatic <strong>and</strong> symptomaticinfection occurs at all ages, including the elderly, possiblybecause of small variations in antigenic specificity or thetransient nature of immunity. In the Tecumseh familiesHCV-OC43HCV-229ENumber of respiratory illnesses1009050080400Number of coronavirus seroconversions322824201612840*000F W S S F W S S F W S S F W S S F W S S F W S S F W S S F W S S F WF W S S1960–61 61–62 62–63 63–64 64–65 65–66 66–67 67–68 68–Dec Total* HCV-OC43 diagnoses were not continued past fall of 1967FIGURE <strong>189</strong>–2 ■ Seasonal distribution of seroconversion to human coronaviruses OC43 <strong>and</strong> 229E, 1960 through 1968, in a group ofchildren’s cottages in Atlanta, Georgia (120 to 175 children 5 to 19 years of age). Forty-one percent of the seroconversions were associatedwith respiratory illness. (Adapted from Kaye, H. S., Marsh, H. B., <strong>and</strong> Dowdle, W. R.: Seroepidemiologic survey of coronavirus [strainOC43] related infections in a children’s population. Am. J. Epidemiol. 94:43–49, 1971; <strong>and</strong> Kaye, H. S., <strong>and</strong> Dowdle, W. R.:Seroepidemiologic survey of coronavirus [strain 229E] infections in a population of children. Am. J. Epidemiol. 101:238–244, 1975.)** **70605040302010Number of respiratory illnessesNumber of coronavirus seroconversions16014012010080604020300200100Number of respiratory illnesses
CHAPTER <strong>189</strong> <strong>Coronaviruses</strong> <strong>and</strong> <strong>Toroviruses</strong>, <strong>Including</strong> <strong>Severe</strong> <strong>Acute</strong> Respiratory Syndrome (SARS) 2383studied during a community-wide HCoV-229E epidemic in1967, only 3 of 54 infections occurred in children youngerthan 4 years of age. The attack rate then rose to a peak of 14percent in individuals 15 to 29 years of age <strong>and</strong> subsequentlyfell with increasing age. 17 The results were different in anoutbreak of HCoV-OC43 infection. With this virus, the highestrate, 29 percent, occurred in children 4 years of age oryounger, <strong>and</strong> rates decreased very little even into adultyears, when the incidence was 22 percent. 71Several recent studies have emphasized the importanceof coronavirus infection in the elderly <strong>and</strong> the burden ofinfection, particularly in individuals in chronic care facilities<strong>and</strong> those with underlying cardiopulmonary disease. 78TRANSMISSIONHuman volunteers can be infected readily via nosedrops,<strong>and</strong> a typical cold can develop 2 to 3 days later; therefore,natural infections are assumed to occur through the respiratoryroute. Monto favors aerosol transmission of HCoVbecause like influenza viruses, HCoVs often cause sharp <strong>and</strong>widespread outbreaks. 70 Whatever the means of transmission,HCoVs do not spread easily, at least within families. 17Nosocomial transmission of coronavirus respiratoryinfection has been reported. The study took place in a neonatalintensive care unit, where prospective surveillance byimmunofluorescence testing of nasal aspirates performed for15 months detected 10 infections with coronavirus OC43, allof them nosocomially acquired. 95 All infants had apnea,bradycardia, or abdominal distention, or a combination ofthese pathologic effects, at the time of infection. Stool sampleswere not examined. The mode of transmission was notdemonstrated in this study.Infection <strong>and</strong> ImmunityPATHOGENESIS, INCUBATION PERIOD, ANDSEROLOGIC RESPONSEIn healthy adults, HCoVs seem to replicate only in the upperrespiratory tract <strong>and</strong> to produce little direct cytopathology.In human embryonic tracheal organ culture, a decline in ciliaryactivity after serial passage was the only cytopathiceffect observed. 64, 104 In contrast to influenza virus <strong>and</strong> adenovirusinfections, which destroy cells in this system, HCoV-229E <strong>and</strong> rhinoviruses had no effect, even though theseviruses were replicating. 112 Very similar events appear totake place in vivo. Electron microscopy of nasal epithelialbiopsy specimens from a young girl with chronic rhinitis <strong>and</strong>bronchitis showed preservation of cellular structures <strong>and</strong>cilia despite replication of coronavirus particles. 168, 77HCoVs have been detected in nasopharyngeal cells,<strong>and</strong> HCoV-229E virus titers from 10 to more than 1000TCID 50 (median tissue culture infective dose) were found fora week or more in nasopharyngeal washings. 75 Bende <strong>and</strong>associates studied the course of HCoV-229E colds in 24 volunteers<strong>and</strong> delineated the typical signs, symptoms, <strong>and</strong>virus shedding patterns; 8 volunteers were asymptomatic. 6The incubation period of HCoV colds is, on average, 2 days,<strong>and</strong> they usually last approximately 1 week. 8Little is known about the pathogenesis or immunity ofHCoV or torovirus infection of the gastrointestinal tract.REINFECTIONRepeated infection with HCoVs is a very common occurrence.Among Atlanta children who seroconverted toHCoV-229E, 35 percent had pre-infection antibody titers of1:10 or higher, <strong>and</strong> 8 percent had titers of 1:20 or higher. 41Antibody against HCoV-OC43 also is a common finding inacute sera. 42 No evidence has shown, however, that antibodyameliorates the clinical illness. 71Nonetheless, evidence from human volunteer experimentsdemonstrates that strain-specific antibody can be protective.Reed infected volunteers with one of several HCoV-229E–like viruses. She found that immunity to homotypicchallenge endured for at least 1 year, but that immunity toheterotypic HCoV-229E strains in these same volunteerswas much lower. 87 IgA antibody may play an importantprotective role. 12Little is known about the role of cell-mediated immunityin resistance to infection with HCoV. In mice, cytotoxic Tcells are generated to the mouse hepatitis virus N protein,<strong>and</strong> immunoreactive lymphocytes probably play a role inboth virus clearance <strong>and</strong> the pathogenesis of neurologic disease.101 HCoV-229E will replicate in human macrophages, 80but the role of these cells <strong>and</strong> cell-mediated immunity in limitinginfection is not understood at present.Clinical Manifestations of RespiratoryTract InfectionsTHE COMMON COLD AND OTHER UPPERRESPIRATORY TRACT ILLNESSESA significant association of HCoVs with respiratory illnesses—most of them cold-like—has been demonstrated inprospective studies of adults <strong>and</strong> families with children.In the Chicago medical students described earlier, HCoV-229E attack rates were 31 percent during “illness periods”<strong>and</strong> only 9 percent in “wellness periods” (p < .001); the illnessesdid not differ significantly from undifferentiated acuterespiratory tract infections caused by respiratory syncytialvirus, parainfluenza viruses, or rhinoviruses. 30 These findingshave been confirmed in several other epidemiologic17, 41, 42studies.Symptoms in the adults in the two investigations citedearlier were much like those in human volunteers inoculatedwith HCoV. 6, 8, 12, 13, 87 Infected volunteers contracted typicalcommon colds, with perhaps more rhinorrhea than occurs inrhinovirus colds; sore throat, cough, malaise, <strong>and</strong> headachewere noted in approximately 50 percent of volunteers.Twenty percent had fever.The proportion of total respiratory disease or colds attributableto HCoV varies markedly by season <strong>and</strong> from year toyear. In the 8.5-year-long Atlanta study (see Fig. <strong>189</strong>–2 <strong>and</strong>Table <strong>189</strong>–1), 7 percent of all respiratory disease was associatedwith HCoV seroconversion, but in the winter of theepidemic year 1961 to 1962, approximately 16 percent ofrespiratory illness was associated with HCoV. Most surveysconclude that approximately 8 to 10 percent of all coldsare associated with serologically detectable coronavirusinfection. 58The possible role of coronaviruses in the etiology ofotitis media with effusion has been the subject of severalrecent studies using polymerase chain reaction (PCR) todetect viral nucleic acid in both nasal secretions <strong>and</strong>middle ear fluid. In one study, 92 children with acute otitismedia were investigated. Coronavirus sequences werefound in 16 children (17%), in the nasopharynx in 14 <strong>and</strong>in middle ear fluid in 7. This prevalence was less than thatof both respiratory syncytial virus (26%) <strong>and</strong> rhinoviruses(32%). 83 In another study of middle ear fluid at the time oftube placement, coronavirus sequences rarely were found(only 3 of 100). 82
2384 SECTION XVII Viral InfectionsTABLE <strong>189</strong>–1 ■ CLINICAL FINDINGS IN 61 AND 43 ATLANTA, GEORGIA, CHILDREN* WHOSEROCONVERTED † TO HCV-229E AND HCV-OC43, RESPECTIVELY, 1960 TO 1968Virus (%) Virus (%)Initial Complaints 229E OC43 Physical Findings 229E OC43Sore throat 66 30 Pharyngeal injection 82 72Coryza 52 19 Coryza 64 49Cough 43 30 Fever, 37.6°C (99.6°F) <strong>and</strong> higher 34 40Fever 21 9Headache 15 NR Fever, 39°C (102.2°F) <strong>and</strong> higher 8 21Cervical adenitis 30 35Pulmonary rales (or dullness) NR 5Rash NR 2*A changing population of 120 to 175 white children 5 to 19 years of age (median, 9 to 11 years of age) in a churchsponsoredhome, 1960 to 1968. The children were housed in cottages of 8 to 12 persons, assigned on the basis of age <strong>and</strong>gender.† Paired acute <strong>and</strong> convalescent sera (2 to 3 weeks) showing fourfold or greater antibody rises by indirect hemagglutination(HCV-229E) or hemagglutination inhibition (HCV-OC43).NR, not reported.Adapted from Kaye, H.S., Marsh, H.B., <strong>and</strong> Dowdle, W.R.: Seroepidemiologic survey of coronavirus (strain OC43) relatedinfections in a children’s population. Am. J. Epidemiol. 94:43–49, 1971; <strong>and</strong> Kaye, H.S., <strong>and</strong> Dowdie, W.R.: Seroepidemiologicsurvey of coronavirus (strain 229E) infections in a population of children. Am. J. Epidemiol. 101:238–244, 1975.LOWER RESPIRATORY TRACT DISEASEAsthma <strong>and</strong> Recurrent WheezingSubstantial evidence indicates that HCoVs can precipitateasthma attacks. 35, 37, 65, 69, 79, 86 In a 2-year surveillance (1967to 1969) of 32 mostly atopic children 1 to 5 years of age hospitalizedin Denver for severe, recurrent bouts of wheezing,19 HCoV infections were diagnosed, 16 of them in a typicalsharp HCoV epidemic. 65 Six children were infected simultaneouslywith either parainfluenza virus or respiratorysyncytial virus. Of the remaining 13 patients, all were symptomatic:3 had mild wheezing, <strong>and</strong> 7 had acute asthmaattacks, 2 of which required intravenous therapy. Threepatients had pneumonia accompanied by radiographicchanges, <strong>and</strong> four were febrile (>38° C). HCoVs were not aslikely to cause wheezing as respiratory syncytial virus was<strong>and</strong> were more likely to cause only cold-like illnesses <strong>and</strong>fewer fevers. These findings have been confirmed in severalother studies. 69Pneumonia <strong>and</strong> Other <strong>Severe</strong> Lower RespiratoryTract InfectionsEvidence of the etiologic involvement of HCoVs in childrenhospitalized for lower respiratory tract disease has beenmore difficult to obtain. For example, among pediatricpatients with lower respiratory tract disease at Children’sHospital, Washington, D.C., the incidence of HCoV in illchildren, 3.5 percent, was actually lower than that in controlpatients with nonrespiratory disease, 8.2 percent. 67 In amore recent study of hospitalized patients with acute respiratorydisease, HCoV infection was found in only 2 of 83infants younger than 5 years of age in whom paired serawere available. 23 Better evidence of causation was found in astudy of infants hospitalized for lower respiratory tract diseaseduring 1967 to 1970. 63 Infections with both HCoV-229E<strong>and</strong> HCoV-OC43 were noted, <strong>and</strong> HCoV was the third mostfrequently occurring virus (behind respiratory syncytialvirus <strong>and</strong> parainfluenza virus 3), both in incidence <strong>and</strong> inspecific association with pneumonia <strong>and</strong> bronchiolitis (Table<strong>189</strong>–2). Many of these infants required oxygen. HCoV-229Ewas cultivated from oropharyngeal swabs from two of theinfants with pneumonia. In newborns, apnea <strong>and</strong> bradycardia94, 95have been described during coronavirus infection.HCoV-associated lower respiratory tract disease has beendetected in adults. A sharp outbreak of acute respiratory diseasesufficient to cause hospitalization of U.S. Marinerecruits was attributed at least partially to HCoV-OC43. 111The clearest association is seen in the elderly with cardiac orchronic obstructive pulmonary disease, in whom infectioncommonly is associated with lower respiratory tract symptoms,although they rarely lead to hospitalization or11, 25, 29, 96, 107death.TABLE <strong>189</strong>–2 ■ RELATIVE INCIDENCE OF VARIOUSRESPIRATORY VIRUS INFECTIONS IN INFANTSWITH PNEUMONIA OR BRONCHIOLITIS,COOK COUNTY HOSPITAL, CHICAGO,ILLINOIS, 1967 TO 1970Number of Infants with IndicatedCondition Who Were Positive for Virus (%)Virus Pneumonia Bronchiolitis TotalRespiratory syncytial 58 (25.1) 48 (32.2) 106 (27.9)Parainfluenza 3 54 (23.4) 28 (18.8) 82 (21.6)Coronavirus* 20 (8.7) 10 (6.7) 30 (7.9)Adenovirus 11 (4.8) 15 (10.1) 26 (6.8)Parainfluenza 1 11 (4.8) 9 (6.0) 20 (5.3)Influenza A 10 (4.3) 5 (3.4) 15 (4.0)Rhinovirus 6 (2.6) 2 (1.3) 8 (2.1)Parainfluenza 2 3 (1.3) 3 (2.0) 6 (1.6)Total 126 (55.0) 83 (55.7) 209Note: Infants were considered positive if virus was recovered or if theyhad serologic evidence of infection; 231 infants with pneumonia <strong>and</strong> 149infants with bronchiolitis were tested. Some infants were positive for morethan one virus.*Both HCV-229E <strong>and</strong> HCV-OC43.From McIntosh, K., Chao, R.K., Krause, H.E., et al.: Coronavirus infection inacute lower respiratory tract disease of infants. J. Infect. Dis. 130:502–507,1974.
CHAPTER <strong>189</strong> <strong>Coronaviruses</strong> <strong>and</strong> <strong>Toroviruses</strong>, <strong>Including</strong> <strong>Severe</strong> <strong>Acute</strong> Respiratory Syndrome (SARS) 2385Enteric Human Coronavirus <strong>and</strong>Torovirus InfectionsCORONAVIRUSESFrom the earliest descriptions of HCoVs, because of theprominence of coronaviruses as a cause of diarrhea in youngcalves <strong>and</strong> pigs, attempts have been made to find coronavirusesin the stool <strong>and</strong> associate them with enteric disease.The particles that have been seen, often called coronaviruslikeparticles (CVLPs), have appeared as frequently in stoolsfrom well subjects as in those with diarrhea in many studies,<strong>and</strong> at times they have been difficult to distinguish from cellularmembrane fragments. Indeed, their authenticity as21, 55, 72, 81viruses has been called into doubt on occasion.This situation has been confused further by the morerecent separation of toroviruses from enteric coronaviruses,although this separation probably will lead ultimately to clarificationof this murky field. Many of the specimens that wereconsidered to contain CVLPs in earlier papers may very wellhave contained toroviruses. At present, electron microscopiststhink that these virus genera can be distinguished in mostinstances on the basis of morphology alone, 22 <strong>and</strong> in manycases the presence of human toroviruses can be confirmedby serologic testing for stool antigens with bovine antisera4, 44, 47containing bovine torovirus (Breda virus) antibody.The first reports of HCoV as a possible cause of humangastroenteritis appeared in 1975; CVLPs were found by electronmicroscopy in the stools of English adults in threesharp outbreaks of nonbacterial gastroenteritis. 14, 16 In thesame year, CVLPs were reported in the stools of healthyadults in India. 60 These rather contrasting publications fromEngl<strong>and</strong> <strong>and</strong> India heralded the beginning of a continuingcontroversy on the etiologic importance of these agents as55, 72enteric pathogens.The firmest link of CVLPs to human enteric disease iswith gastroenteritis in the very young, especially neonatalnecrotizing enterocolitis (NEC). A controlled epidemiologicinvestigation of NEC was conducted in two hospitals inFrance, one with <strong>and</strong> one without an NEC outbreak. 18Within each hospital, newborns with “no pathologic occurrence”were used as controls. In the NEC-free hospital, noCVLPs were found in the stools of 21 controls, but 2 patientswith mild diarrhea had CVLPs. In the NEC hospital, 23 ofthe 32 (72%) NEC patients had fecal CVLPs, whereas only 3of 26 (11.5%) controls were positive (p < .02). Similarly,CVLPs were observed in infants who were part of a NECoutbreak in a special care nursery in Texas. 88 This outbreakyielded a virus that subsequently has been adapted togrowth in tissue culture <strong>and</strong> to some extent characterized. 53In September 1979, an episode of acute, severe (bloodystools, bilious gastric aspirates, abdominal distention) gastroenteritisoccurred in a neonatal intensive care unit inArizona, <strong>and</strong> several clinical signs were associated significantlywith CVLPs in patients’ stools. 106 Two children diedin this outbreak, <strong>and</strong> another infant death, with carefulelectron-microscopic description of coronavirus infection inthe distal end of the small bowel, occurred in Oklahoma. 89In Italy, a case-control study of infants <strong>and</strong> young childrenwith enteritis found a significant difference in fecal CVLPpresence between the ill <strong>and</strong> control groups: 16.3 percent inill children <strong>and</strong> 1.6 percent in controls (p
2386 SECTION XVII Viral Infectionspossibility that these isolates were of mouse origin has notbeen excluded. In addition, other studies have failed todemonstrate coronavirus RNA in the central nervous systemtissue of patients with multiple sclerosis 99 or have foundsuch RNA in the same proportion of patients with demyelinatingdiseases as in controls. 20 Further investigation will beneeded to establish whether HCoVs are related causally toany neurologic disease in humans.Laboratory DiagnosisVIRUS ISOLATIONRespiratory <strong>Coronaviruses</strong>Isolation of HCoVs from clinical material has been limited toa few research laboratories. On primary isolation, someHCoV-229E–type strains will replicate <strong>and</strong> produce a cytopathiceffect in secondary human embryo kidney 31 or certaindiploid cell lines: WI-38, MRC-5, <strong>and</strong> MA-177. 30, 38 The cellsbecome “stringy” after several days’ incubation in a rollerdrum at 33° C or on subsequent passage. However, someHCoV-229E–like strains require organ culture. 49, 57, 87 In contrast,all HCoV-OC43–like strains seem to require organ culturefor primary isolation, usually human embryonictrachea or nasal mucosa.Enteric Coronavirus-like ParticlesGrowth of these agents in cell or organ culture has beenreported, 15, 88, 115 <strong>and</strong> one isolate has been adapted to growthin tissue culture. 53<strong>Toroviruses</strong>Human toroviruses have not been grown in any culturesystem.VIRUS DETECTION TECHNIQUESRespiratory <strong>Coronaviruses</strong>Respiratory coronaviruses can be sought successfully inclinical samples by direct antigen-detection techniques orby nucleic acid detection. Some evidence that antigens inHCoV-229E <strong>and</strong> HCoV-OC43 may be representative of therespiratory HCoV group as a whole exists, 57 although thissuggestion is not accepted universally. Both immunofluorescence68, 95 <strong>and</strong> ELISA have been used successfully for the35, 56, 69diagnosis of HCoV infection.The HCoV genome has been detected successfully both byRNA-RNA hybridization <strong>and</strong> by reverse transcription PCR.Hybridization has been reported to detect as little as 10TCID 50 of virus in nasal washes from volunteers infected withHCoV-229E <strong>and</strong> to have high specificity as well. 77 PCR amplificationof both HCoV-229E <strong>and</strong> HCoV-OC43 from respiratorysecretions also has been described <strong>and</strong> applied successfully to76, 82, 83, 93both nasal washings <strong>and</strong> middle ear fluid. Fewresearchers question that these methods will be useful becausethey are sensitive <strong>and</strong> specific. In addition, the PCR methodologycan use primers that are specific to the HCoV group, butit may pick up infections caused by strains that might be64, 66related only distantly to the two prototype strains.Enteric <strong>Coronaviruses</strong>Enteric coronaviruses have been detected primarily by electronmicroscopy of negatively stained preparations fromclarified stool specimens. The possible confusion of coronaviruses<strong>and</strong> toroviruses in such preparations has been discussedearlier. Immune electron microscopy has beenhelpful in identifying such viruses in purified preparations,but not directly in stool samples.An antigen-detection ELISA for enteric coronavirus hasbeen described <strong>and</strong> applied to fecal specimens from healthy<strong>and</strong> diarrheal subjects in Thail<strong>and</strong>. 51, 52 Findings were similarto those of the electron-microscopic stool studies from India:coronavirus excretion was higher in healthy subjects, <strong>and</strong>excretion of viral antigen was observed occasionally to persistfor months. With the adaptation of an antigenically distinctstrain of human enteric coronavirus (HEC) to cell culture,constructing antigen-detection systems for further epidemiologic<strong>and</strong> clinical study of this virus should be possible. 53<strong>Toroviruses</strong>As with enteric coronaviruses, the primary detectionmethod for toroviruses is electron microscopy of clarifiedfecal specimens. Torovirus identity can be specified furtherby either immune electron microscopy or antigen-detectionELISA with the use of antisera prepared against the Bredavirus of calves. 4, 44, 45 With increasing experience, electronmicroscopists can differentiate toroviruses from enteric22, 36coronaviruses on the basis of morphology alone.SERODIAGNOSISFor respiratory coronaviruses, antigens can be preparedwith the use of tissue culture–grown virus or suckling mousebrain preparations. Complement fixation, 17, 63, 67, 71, 90 hemagglutinationinhibition (for HCoV-OC43 only), 42 neutralization,23 ELISA, 13, 28, 46, 56 indirect hemagglutination, 41 <strong>and</strong>Western blot 84 all have been used.Serodiagnostic techniques specific for enteric coronaviruseshave not been described. Serodiagnosis oftorovirus infections has been performed both by immuneelectron microscopy <strong>and</strong> by hemagglutination inhibition. 22Prevention <strong>and</strong> TreatmentEven if preparing sufficient antigen for vaccine preparationwere possible, the high reinfection rate with these virusessuggests that a vaccine may be ineffective in preventingHCoV-caused respiratory illness. Ribavirin has some in vitroactivity against coronavirus <strong>and</strong> has been shown to reducehepatitis in mice infected with mouse hepatitis virus, a coronavirusantigenically related to HCoV-OC43. 97AcknowledgmentDr. McIntosh gratefully acknowledges the support of The Bruce R. <strong>and</strong> JoleneM. McCraw Fund.REFERENCES1. Afzelius, B. A.: Ultrastructure of human nasal epithelium during anepisode of coronavirus infection. Virchows Arch. 424:295–300, 1994.2. Almeida, J. D., <strong>and</strong> Tyrrell, D. A. J.: The morphology of three previouslyuncharacterized human respiratory viruses that grow in organ culture.J. Gen. Virol. 1:175–178, 1967.3. Arbour, N., Day, R., Newcombe, J., <strong>and</strong> Talbot, P. J.: Neuroinvasion byhuman respiratory coronaviruses. J. Virol. 74:8913–8921, 2000.4. Beards, G. M., Brown, D. W., Green, J., <strong>and</strong> Flewett, T. H.: Preliminarycharacterisation of torovirus-like particles of humans: Comparison withBerne virus of horses <strong>and</strong> Breda virus of calves. J. Med. Virol. 20:67–78,1986.
CHAPTER <strong>189</strong> <strong>Coronaviruses</strong> <strong>and</strong> <strong>Toroviruses</strong>, <strong>Including</strong> <strong>Severe</strong> <strong>Acute</strong> Respiratory Syndrome (SARS) 23875. Beards, G. M., Hall, C., Green, J., et al.: An enveloped virus in stools ofchildren <strong>and</strong> adults with gastroenteritis that resembles the Breda virus ofcalves. Lancet 1:1050–1052, 1984.6. Bende, M., Barrow, I., Heptonstall, J., et al.: Changes in human nasalmucosa during experimental coronavirus common colds. Acta Otolaryngol.107:262–269, 1989.7. Bradburne, A. F.: Antigenic relationships amongst coronaviruses. Arch.Gesamte Virusforsch. 31:352–364, 1970.8. Bradburne, A. F., Bynoe, M. L., <strong>and</strong> Tyrrell, D. A.: Effects of a “new”human respiratory virus in volunteers. B. M. J. 3:767–769, 1967.9. Bradburne, A. F., <strong>and</strong> Somerset, B. A.: Coronavirus antibody titres in seraof healthy adults <strong>and</strong> experimentally infected volunteers. J. Hyg. (Lond.)70:235–244, 1972.10. Burks, J. S., DeVald, B. L., Jankovsky, L. D., <strong>and</strong> Gerdes, J. C.: Two coronavirusesisolated from central nervous system tissue of two multiplesclerosis patients. Science 209:933–934, 1980.11. Buscho, R. O., Saxtan, D., Shultz, P. S., et al.: Infections with viruses <strong>and</strong>Mycoplasma pneumoniae during exacerbations of chronic bronchitis.J. Infect. Dis. 137:377–383, 1978.12. Callow, K. A.: Effect of specific humoral immunity <strong>and</strong> some non-specificfactors on resistance of volunteers to respiratory coronavirus infection.J. Hyg. (Lond.) 95:173–<strong>189</strong>, 1985.13. Callow, K. A., Parry, H. F., Sergeant, M., <strong>and</strong> Tyrrell, D. A.: The timecourse of the immune response to experimental coronavirus infection ofman. Epidemiol. Infect. 105:435–446, 1990.14. Caul, E. O., <strong>and</strong> Clarke, S. K.: Coronavirus propagated from patient withnon-bacterial gastroenteritis. Lancet 2:953–954, 1975.15. Caul, E. O. <strong>and</strong> Egglestone, S. I.: Further studies on human enteric coronaviruses.Arch. Virol. 54:107–117, 1977.16. Caul, E. O., Paver, W. K., <strong>and</strong> Clarke, S. K.: Coronavirus particlesin faeces from patients with gastroenteritis. Letter. Lancet. 1:1192,1975.17. Cavallaro, J. J., <strong>and</strong> Monto, A. S.: Community-wide outbreak of infectionwith a 229E-like coronavirus in Tecumseh, Michigan. J. Infect. Dis.122:272–279, 1970.18. Chany, C., Moscovici, O., Lebon, P., <strong>and</strong> Rousset, S.: Association of coronavirusinfection with neonatal necrotizing enterocolitis. Pediatrics69:209–214, 1982.19. Cornelissen, L. A., Wierda, C. M., van der Meer, F. J., et al.: Hemagglutininesterase,a novel structural protein of torovirus. J. Virol. 71:5277–5286,1997.20. Dessau, R. B., Lisby, G., <strong>and</strong> Frederiksen, J. L.: <strong>Coronaviruses</strong> in spinalfluid of patients with acute monosymptomatic optic neuritis. Acta Neurol.Sc<strong>and</strong>. 100:88–91, 1999.21. Dourmashkin, R. R., Davies, H. A., Smith, H., <strong>and</strong> Bird, R. G.: Arecoronavirus-like particles seen in diarrhoea stools really viruses? Lancet2:971–972, 1980.22. Duckmanton, L., Luan, B., Devenish, J., et al.: Characterization oftorovirus from human fecal specimens. Virology 239:158–168, 1997.23. El-Sahly, H. M., Atmar, R. L., Glezen, W. P., <strong>and</strong> Greenberg, S. B.: Spectrumof clinical illness in hospitalized patients with “common cold” virusinfections. Clin. Infect. Dis. 31:96–100, 2000.24. Falsey, A. R., McCann, R. M., Hall, W. J., et al.: The “common cold” infrail older persons: Impact of rhinovirus <strong>and</strong> coronavirus in a senior daycarecenter. J. Am. Geriatr. Soc. 45:706–711, 1997.25. Falsey, A. R., McCann, R. M., Hall, W. J., et al.: <strong>Acute</strong> respiratory tractinfection in daycare centers for older persons. J. Am. Geriatr. Soc.43:30–36, 1995.26. Gerdes, J. C., Klein, I., DeVald, B. L., <strong>and</strong> Burks, J. S.: Coronavirus isolatesSK <strong>and</strong> SD from multiple sclerosis patients are serologically relatedto murine coronaviruses A59 <strong>and</strong> JHM <strong>and</strong> human coronavirus OC43, butnot to human coronavirus 229E. J. Virol. 38:231–238, 1981.27. Gerna, G., Passarani, N., Battaglia, M., <strong>and</strong> Rondanelli, E. G.: Humanenteric coronaviruses: Antigenic relatedness to human coronavirus OC43<strong>and</strong> possible etiologic role in viral gastroenteritis. J. Infect. Dis. 151:796–803, 1985.28. Gill, E. P., Dominguez, E. A., Greenberg, S. B., et al.: Development <strong>and</strong>application of an enzyme immunoassay for coronavirus OC43 antibody inacute respiratory illness. J. Clin. Microbiol. 32:2372–2376, 1994.29. Gump, D. W., Phillips, C. A., Forsyth, B. R., et al.: Role of infection inchronic bronchitis. Am. Rev. Respir. Dis. 113:465–474, 1976.30. Hamre, D., <strong>and</strong> Beem, M.: Virologic studies of acute respiratory disease inyoung adults. V. Coronavirus 229E infections during six years of surveillance.Am. J. Epidemiol. 96:94–106, 1972.31. Hamre, D., <strong>and</strong> Procknow, J. J.: A new virus isolated from the human respiratorytract. Proc. Soc. Exp. Biol. Med. 121:190–193, 1966.32. Hasony, H. J., <strong>and</strong> Macnaughton, M. R.: Prevalence of human coronavirusantibody in the population of southern Iraq. J. Med. Virol.9:209–216, 1982.33. Hendley, J. O., Fishburne, H. B., <strong>and</strong> Gwaltney, J. M.: Coronavirus infectionsin working adults. Eight-year study with 229 E <strong>and</strong> OC 43. Am. Rev.Respir. Dis. 105:805–811, 1972.34. Herold, J., Raabe, T., <strong>and</strong> Siddell, S.: Molecular analysis of the humancoronavirus (strain 229E) genome. Arch. Virol. Suppl. 7:63–74, 1993.35. Isaacs, D., Flowers, D., Clarke, J. R., et al.: Epidemiology of coronavirusrespiratory infections. Arch. Dis. Child. 58:500–503, 1983.36. Jamieson, F. B., Wang, E. E., Bain, C., et al.: Human torovirus: A newnosocomial gastrointestinal pathogen. J. Infect. Dis. 178:1263–1269,1998.37. Johnston, S. L., Pattemore, P. K., S<strong>and</strong>erson, G., et al.: Community studyof role of viral infections in exacerbations of asthma in 9–11 year old children.B. M. J. 310:1225–1229, 1995.38. Kapikian, A. Z., James, H. D., Kelly, S. J., et al.: Isolation from man of“avian infectious bronchitis virus–like” viruses (coronaviruses) similar to229E virus, with some epidemiological observations. J. Infect. Dis. 119:282–290, 1969.39. Kapikian, A. Z., James, H. D., Jr., Kelly, S. J., et al.: Hemadsorption bycoronavirus strain OC43. Proc. Soc. Exp. Biol. Med. 139:179–186, 1972.40. Kaye, H. S., <strong>and</strong> Dowdle, W. R.: Some characteristics of hemagglutinationof certain strains of “IBV-like” virus. J. Infect. Dis. 120:576–581,1969.41. Kaye, H. S., <strong>and</strong> Dowdle, W. R.: Seroepidemiologic survey of coronavirus(strain 229E) infections in a population of children. Am. J. Epidemiol.101:238–244, 1975.42. Kaye, H. S., Marsh, H. B., <strong>and</strong> Dowdle, W. R.: Seroepidemiologic surveyof coronavirus (strain OC 43) related infections in a children’s population.Am. J. Epidemiol. 94:43–49, 1971.43. Kidd, A. H., Esrey, S. A., <strong>and</strong> Ujfalusi, M. J.: Shedding of coronavirus-likeparticles by children in Lesotho. J. Med. Virol. 27:164–169, 1989.44. Koopmans, M., Petric, M., Glass, R. I., <strong>and</strong> Monroe, S. S.: Enzyme-linkedimmunosorbent assay reactivity of torovirus-like particles in fecalspecimens from humans with diarrhea. J. Clin. Microbiol. 31:2738–2744,1993.45. Koopmans, M. P., Goosen, E. S., Lima, A. A., et al.: Association oftorovirus with acute <strong>and</strong> persistent diarrhea in children. Pediatr. Infect.Dis. J. 16:504–507, 1997.46. Kraaijeveld, C. A., Reed, S. E., <strong>and</strong> Macnaughton, M. R.: Enzyme-linkedimmunosorbent assay for detection of antibody in volunteers experimentallyinfected with human coronavirus strain 229 E. J. Clin. Microbiol.12:493–497, 1980.47. Krishnan, T., <strong>and</strong> Naik, T. N.: Electron-microscopic evidence of toroviruslike particles in children with diarrhoea. Indian J. Med. Res. 105:108–110,1997.48. Lai, M. M., <strong>and</strong> Holmes, K. V.: Coronaviridae: The viruses <strong>and</strong> their replication.In Knipe, D. M., Howley, P. M., Griffin, D. E., et al. (eds.): FieldsVirology. Philadelphia, Lippincott-Raven, 2001.49. Larson, H. E., Reed, S. E., <strong>and</strong> Tyrrell, D. A.: Isolation of rhinoviruses <strong>and</strong>coronaviruses from 38 colds in adults. J. Med. Virol. 5:221–229, 1980.50. Lee, H. J., Shieh, C. K., Gorbalenya, A. E., et al.: The complete sequence(22 kilobases) of murine coronavirus gene 1 encoding the putative proteases<strong>and</strong> RNA polymerase. Virology 180:567–582, 1991.51. Leechanachai, P., Yoosook, C., <strong>and</strong> Matangkasombut, P.: Epidemiologicalstudy of enteric coronavirus excretion by an enzyme-linked immunosorbentassay. J. Med. Assoc. Thai. 72:452–457, 1989.52. Leechanachai, P., Yoosook, C., Saguanwongse, S., <strong>and</strong> Matangkasombut,P.: Comparison of a modified enzyme-linked immunosorbent assay withimmunosorbent electron microscopy to detect coronavirus in humanfaecal specimens. J. Diarrhoeal Dis. Res. 5:24–29, 1987.53. Luby, J. P., Clinton, R., <strong>and</strong> Kurtz, S.: Adaptation of human enteric coronavirusto growth in cell lines. J. Clin. Virol. 12:43–51, 1999.54. Macnaughton, M. R.: Occurrence <strong>and</strong> frequency of coronavirus infectionsin humans as determined by enzyme-linked immunosorbent assay. Infect.Immun. 38:419–423, 1982.55. Macnaughton, M. R., <strong>and</strong> Davies, H. A.: Human enteric coronaviruses.Brief review. Arch. Virol. 70:301–313, 1981.56. Macnaughton, M. R., Flowers, D., <strong>and</strong> Isaacs, D.: Diagnosis of humancoronavirus infections in children using enzyme-linked immunosorbentassay. J. Med. Virol. 11:319–325, 1983.57. Macnaughton, M. R., Madge, M. H., <strong>and</strong> Reed, S. E.: Two antigenic groupsof human coronaviruses detected by using enzyme-linked immunosorbentassay. Infect. Immun. 33:734–737, 1981.58. Makela, M. J., Puhakka, T., Ruuskanen, O., et al.: Viruses <strong>and</strong> bacteria inthe etiology of the common cold. J. Clin. Microbiol. 36:539–542, 1998.59. Marshall, J. A., Birch, C. J., Williamson, H. G., et al.: Coronavirus-likeparticles <strong>and</strong> other agents in the faeces of children in Efate, Vanuatu. J.Trop. Med. Hyg. 85:213–215, 1982.60. Mathan, M., Mathan, V. I., Swaminathan, S. P., <strong>and</strong> Yesudoss, S.: Pleomorphicvirus-like particles in human faeces. Lancet 1:1068–1069, 1975.61. McIntosh, K.: <strong>Coronaviruses</strong>: A comparative review. Curr. Top. Microbiol.Immunol. 63:83–129, 1974.62. McIntosh, K., Becker, W. B., <strong>and</strong> Chanock, R. M.: Growth in sucklingmousebrain of “IBV-like” viruses from patients with upper respiratorytract disease. Proc. Natl. Acad. Sci. U.S.A. 58:2268–2273, 1967.63. McIntosh, K., Chao, R. K., Krause, H. E., et al.: Coronavirus infection inacute lower respiratory tract disease of infants. J. Infect. Dis.130:502–507, 1974.64. McIntosh, K., Dees, J. H., Becker, W. B., et al.: Recovery in tracheal organcultures of novel viruses from patients with respiratory disease. Proc.Natl. Acad. Sci. U.S.A. 57:933–940, 1967.65. McIntosh, K., Ellis, E. F., Hoffman, L. S., et al.: Association of viral <strong>and</strong>bacterial respiratory infection with exacerbations of wheezing in youngasthmatic children. Chest 63(Suppl.):43, 1973.
2388 SECTION XVII Viral Infections66. McIntosh, K., Kapikian, A. Z., Hardison, K. A., et al.: Antigenic relationshipsamong the coronaviruses of man <strong>and</strong> between human <strong>and</strong> animalcoronaviruses. J. Immunol. 102:1109–1118, 1969.67. McIntosh, K., Kapikian, A. Z., Turner, H. C., et al.: Seroepidemiologicstudies of coronavirus infection in adults <strong>and</strong> children. Am. J. Epidemiol.91:585–592, 1970.68. McIntosh, K., McQuillin, J., Reed, S. E., <strong>and</strong> Gardner, P. S.: Diagnosis ofhuman coronavirus infection by immunofluorescence: Method <strong>and</strong> applicationto respiratory disease in hospitalized children. J. Med. Virol.2:341–346, 1978.69. Mertsola, J., Ziegler, T., Ruuskanen, O., et al.: Recurrent wheezy bronchitis<strong>and</strong> viral respiratory infections. Arch. Dis. Child. 66:124–129, 1991.70. Monto, A. S.: Medical reviews. <strong>Coronaviruses</strong>. Yale J. Biol. Med.47:234–251, 1974.71. Monto, A. S., <strong>and</strong> Lim, S. K.: The Tecumseh study of respiratory illness.VI. Frequency of <strong>and</strong> relationship between outbreaks of coronavirusinfection. J. Infect. Dis. 129:271–276, 1974.72. Mortensen, M. L., Ray, C. G., Payne, C. M., et al.: Coronaviruslike particlesin human gastrointestinal disease. Epidemiologic, clinical, <strong>and</strong> laboratoryobservations. Am. J. Dis. Child. 139:928–934, 1985.73. Murray, R. S., Brown, B., Brian, D., <strong>and</strong> Cabirac, G. F.: Detection of coronavirusRNA <strong>and</strong> antigen in multiple sclerosis brain. Ann. Neurol.31:525–533, 1992.74. Murray, R. S., Cai, G. Y., Hoel, K., et al.: Coronavirus infects <strong>and</strong> causesdemyelination in primate central nervous system. Virology 188:274–284,1992.75. Myint, S., Harmsen, D., Raabe, T., <strong>and</strong> Siddell, S. G.: Characterization ofa nucleic acid probe for the diagnosis of human coronavirus 229E infections.J. Med. Virol. 31:165–172, 1990.76. Myint, S., Johnston, S., S<strong>and</strong>erson, G., <strong>and</strong> Simpson, H.: Evaluation ofnested polymerase chain methods for the detection of human coronaviruses229E <strong>and</strong> OC43. Mol. Cell. Probes 8:357–364, 1994.77. Myint, S., Siddell, S., <strong>and</strong> Tyrrell, D.: Detection of human coronavirus229E in nasal washings using RNA:RNA hybridisation. J. Med. Virol.29:70–73, 1989.78. Nicholson, K. G., Kent, J., Hammersley, V., <strong>and</strong> Cancio, E.: <strong>Acute</strong> viralinfections of upper respiratory tract in elderly people living in the community:Comparative, prospective, population based study of diseaseburden. B. M. J. 315:1060–1064, 1997.79. Pattemore, P. K., Johnston, S. L., <strong>and</strong> Bardin, P. G.: Viruses as precipitantsof asthma symptoms. I. Epidemiology. Clin. Exp. Allergy22:325–336, 1992.80. Patterson, S., <strong>and</strong> Macnaughton, M. R.: Replication of human respiratorycoronavirus strain 229E in human macrophages. J. Gen. Virol.60:307–314, 1982.81. Payne, C. M., Ray, C. G., Borduin, V., et al.: An eight-year study of theviral agents of acute gastroenteritis in humans: Ultrastructural observations<strong>and</strong> seasonal distribution with a major emphasis on coronavirus-likeparticles. Diagn. Microbiol. Infect. Dis. 5:39–54, 1986.82. Pitkaranta, A., Jero, J., Arruda, E., et al.: Polymerase chainreaction–based detection of rhinovirus, respiratory syncytial virus,<strong>and</strong> coronavirus in otitis media with effusion. J. Pediatr. 133:390–394,1998.83. Pitkaranta, A., Virolainen, A., Jero, J., et al.: Detection of rhinovirus, respiratorysyncytial virus, <strong>and</strong> coronavirus infections in acute otitis mediaby reverse transcriptase polymerase chain reaction. Pediatrics102:291–295, 1998.84. Pohl-Koppe, A., Raabe, T., Siddell, S. G., <strong>and</strong> ter Meulen, V.: Detection ofhuman coronavirus 229E–specific antibodies using recombinant fusionproteins. J. Virol. Methods 55:175–183, 1995.85. Puel, J. M., Orillac, M. S., Bauriaud, R. M., et al.: Occurrence of virusesin human stools in the Ahaggar (Alberia). J. Hyg. (Lond.) 89:171–174,1982.86. Rakes, G. P., Arruda, E., Ingram, J. M., et al.: Rhinovirus <strong>and</strong> respiratorysyncytial virus in wheezing children requiring emergency care. IgE <strong>and</strong>eosinophil analyses. Am. J. Respir. Crit. Care. Med. 159:785–790, 1999.87. Reed, S. E.: The behaviour of recent isolates of human respiratory coronavirusin vitro <strong>and</strong> in volunteers: Evidence of heterogeneity among 229Erelatedstrains. J. Med. Virol. 13:179–192, 1984.88. Resta, S., Luby, J. P., Rosenfeld, C. R., <strong>and</strong> Siegel, J. D.: Isolation<strong>and</strong> propagation of a human enteric coronavirus. Science 229:978–981,1985.89. Rettig, P. J., <strong>and</strong> Altshuler, G. P.: Fatal gastroenteritis associated withcoronaviruslike particles. Am. J. Dis. Child. 139:245–248, 1985.90. Riski, H., <strong>and</strong> Hovi, T.: Coronavirus infections of man associated withdiseases other than the common cold. J. Med. Virol. 6:259–265, 1980.91. Schnagl, R. D., Holmes, I. H., <strong>and</strong> Mackay-Scollay, E. M.: Coronaviruslikeparticles in Aboriginals <strong>and</strong> non-Aboriginals in Western Australia.Med. J. Aust. 1:307–309, 1978.92. Sitbon, M.: Human-enteric-coronaviruslike particles (CVLP) with differentepidemiological characteristics. J. Med. Virol. 16:67–76, 1985.93. Sizun, J., Arbour, N., <strong>and</strong> Talbot, P. J.: Comparison of immunofluorescencewith monoclonal antibodies <strong>and</strong> RT-PCR for the detection ofhuman coronaviruses 229E <strong>and</strong> OC43 in cell culture. J. Virol. Methods72:145–152, 1998.94. Sizun, J., Soupre, D., Giroux, J. D., et al.: Nasal colonization with coronavirus<strong>and</strong> apnea of the premature newborn. Acta Paediatr. 82:238, 1993.95. Sizun, J., Soupre, D., Legr<strong>and</strong>, M. C., et al.: Neonatal nosocomial respiratoryinfection with coronavirus: A prospective study in a neonatalintensive care unit. Acta Paediatr. 84:617–620, 1995.96. Smith, C. B., Golden, C. A., Kanner, R. E., <strong>and</strong> Renzetti, A. D.: Associationof viral <strong>and</strong> Mycoplasma pneumoniae infections with acute respiratoryillness in patients with chronic obstructive pulmonary diseases.Am. Rev. Respir. Dis. 121:225–232, 1980.97. Smith, R. A., <strong>and</strong> Kirkpatrick, W.: Ribavirin, Broad Spectrum AntiviralAgent. New York, Academic, 1980.98. Snijder, E. J., <strong>and</strong> Horzinek, M. C.: <strong>Toroviruses</strong>: Replication, evolution<strong>and</strong> comparison with other members of the coronavirus-like superfamily.J. Gen. Virol. 74:2305–2316, 1993.99. Sorensen, O., Collins, A., Flintoff, W., et al.: Probing for the human coronavirusOC43 in multiple sclerosis. Neurology 36:1604–1606, 1986.100. Stewart, J. N., Mounir, S., <strong>and</strong> Talbot, P. J.: Human coronavirus geneexpression in the brains of multiple sclerosis patients. Virology191:502–505, 1992.101. Stohlman, S. A., <strong>and</strong> Hinton, D. R.: Viral induced demyelination. BrainPathol. 11:92–106, 2001.102. Tanaka, R., Iwasaki, Y., <strong>and</strong> Koprowski, H.: Intracisternal virus-likeparticles in brain of a multiple sclerosis patient. J. Neurol. Sci.28:121–126, 1976.103. Tyrrell, D. A., Almeida, J. D., Cunningham, C. H., et al.: Coronaviridae.Intervirology 5:76–82, 1975.104. Tyrrell, D. A. J., <strong>and</strong> Bynoe, M. L.: Cultivation of a novel type ofcommon-cold virus in organ cultures. B. M. J. 1:1467–1470, 1965.105. Uziel, Y., Laxer, R. M., <strong>and</strong> Petric, M.: Torovirus gastroenteritis presentingas acute abdomen. Clin. Infect. Dis. 28:925–926, 1999.106. Vaucher, Y. E., Ray, C. G., Minnich, L. L., et al.: Pleomorphic, enveloped,virus-like particles associated with gastrointestinal illness in neonates.J. Infect. Dis. 145:27–36, 1982.107. Walsh, E. E., Falsey, A. R., <strong>and</strong> Hennessey, P. A.: Respiratory syncytial<strong>and</strong> other virus infections in persons with chronic cardiopulmonary disease.Am. J. Respir. Crit. Care Med. 160:791–795, 1999.108. Weiss, M., <strong>and</strong> Horzinek, M. C.: The proposed family Toroviridae:Agents of enteric infections. Brief review. Arch. Virol. 92:1–15, 1987.109. Weiss, M., Steck, F., <strong>and</strong> Horzinek, M. C.: Purification <strong>and</strong> partial characterizationof a new enveloped RNA virus (Berne virus). J. Gen. Virol.64:1849–1858, 1983.110. Weiss, S. R.: <strong>Coronaviruses</strong> SD <strong>and</strong> SK share extensive nucleotidehomology with murine coronavirus MHV-A59, more than that sharedbetween human <strong>and</strong> murine coronaviruses. Virology 126:669–677, 1983.111. Wenzel, R. P., Hendley, J. O., Davies, J. A., <strong>and</strong> Gwaltney, J. M.: Coronavirusinfections in military recruits. Three-year study with coronavirusstrains OC43 <strong>and</strong> 229E. Am. Rev. Respir. Dis. 109:621–624, 1974.112. Winther, B., Gwaltney, J. M., <strong>and</strong> Hendley, J. O.: Respiratory virusinfection of monolayer cultures of human nasal epithelial cells. Am. Rev.Respir. Dis. 141:839–845, 1990.113. Woode, G. N., Reed, D. E., Runnels, P. L., et al.: Studies with an unclassifiedvirus isolated from diarrheic calves. Vet. Microbiol. 7:221–240,1982.114. Yeager, C. L., Ashmun, R. A., Williams, R. K., et al.: Human aminopeptidaseN is a receptor for human coronavirus 229E. Nature 357:420–422,1992.115. Zhang, X. M., Herbst, W., Kousoulas, K. G., <strong>and</strong> Storz, J.: Biological <strong>and</strong>genetic characterization of a hemagglutinating coronavirus isolatedfrom a diarrhoeic child. J. Med. Virol. 44:152–161, 1994.
CHAPTER <strong>189</strong> <strong>Coronaviruses</strong> <strong>and</strong> <strong>Toroviruses</strong>, <strong>Including</strong> <strong>Severe</strong> <strong>Acute</strong> Respiratory Syndrome (SARS) 2389<strong>Severe</strong> acute respiratory syndrome (SARS) is a new infectiousdisease that had its origin in Guangdong Province,China, in the fall of 2002. New information about the epidemiology,etiology, clinical manifestations, <strong>and</strong> treatmentof SARS has been <strong>and</strong> is being gathered at an unprecedentedrate; hence, this chapter, which was written in April 2003,will be incomplete in many areas at the time this book ispublished. However, the pediatric clinical data reflect thefirst-h<strong>and</strong> experience of four of the authors.The syndrome is associated with considerable morbidity<strong>and</strong> mortality, <strong>and</strong> its severity appears to be more marked inadults than in young children.HistoryThe disease first appeared as an apparent outbreak of atypicalpneumonia in Guangdong Province in southern China. 15The first case of SARS occurred in mid-November 2002 inFoshan City. The Chinese Ministry of Health reported 305cases, which included 105 health care workers <strong>and</strong> 5 deaths,occurring between mid-November 2002 <strong>and</strong> February 9,2003, in six municipalities in Guangdong Province.SARS then spread to Hong Kong, where the first recognizedcase was admitted to a hospital on February 22, 2003.The patient was a 64-year-old male physician from ZhongshanUniversity in Guangdong Province, who, before he washospitalized, stayed in a Hong Kong hotel for 1 day. Eightguests who stayed on the same floor <strong>and</strong> two others whostayed on different floors of the hotel acquired the disease,which led to outbreaks of SARS in other countries whenthey returned home or traveled to other countries. Specifically,secondary cases occurred in two Hong Kong hospitals,<strong>and</strong> outbreaks in Singapore, Toronto, <strong>and</strong> Hanoi wereobserved. As of April 16, 2003, a total of 3293 cases ofSARS <strong>and</strong> 159 deaths had been reported to the World HealthOrganization (WHO) from 16 countries. 5EtiologyCHAPTER<strong>189</strong>B<strong>Severe</strong> <strong>Acute</strong> RespiratorySyndrome (SARS)ELLIS K. L. HON ■ ALBERT M. LI ■ EDMUND A. S. NELSON■ CHI WAI W. LEUNG ■ JAMES D. CHERRYPoutanen <strong>and</strong> associates 12 published the results from astudy of 10 adult patients with SARS seen in Toronto,Canada, in March 2003. They carried out an extensivesearch for virus, mycoplasma, chlamydia, rickettsia, bacteria,<strong>and</strong> fungi. Human metapneumovirus was found bynested polymerase chain reaction (PCR) in bronchoalveolarlavage fluid <strong>and</strong> nasopharyngeal swabs from five patients. Anovel coronavirus was isolated in Vero cell cultures of respiratoryspecimens from five patients; four of the five patientswith coronavirus isolations also had positive PCR results forhuman metapneumovirus. Subsequently, a novel coronaviruswas identified in respiratory specimens from patientswith SARS in several countries in WHO network laboratories.4, 16 The coronavirus has a cytopathic effect (CPE) inVero <strong>and</strong> FRnK-4 cells, <strong>and</strong> it can be identified by a reversetranscriptase PCR (RT-PCR) in respiratory specimens. Antibodyto this coronavirus can be detected in sera frompatients by indirect fluorescent antibody (IFA) testing4, 16<strong>and</strong> enzyme-linked immunosorbent assays (ELISA).Hyperimmune sera against numerous animal coronaviruses<strong>and</strong> the human 229E coronavirus inhibit the growth of thisnew coronavirus in tissue culture.This newly discovered coronavirus likely is the causativeagent of SARS. However, the role of other agents as cofactorsis not clear at the present time. Of interest with regardto coronaviruses is that they have a high frequency of recombination.9, 10 Hence, this new coronavirus possibly has arisenby a reassortment of genes between human <strong>and</strong> animal coronavirusesduring a chance simultaneous infection. Possiblerecombinants could be between a human common cold virusstrain, such as 229E or OC43, <strong>and</strong> a pig (PRCoV or HEV),cat (FIPV), mouse (MHV), or cow (BCoV) mammalian respiratorypathogen or an avian strain such as IBV from chickensor TCoV from turkeys.EpidemiologyAs of April 2003, providing an accurate <strong>and</strong> completedescription of the epidemiology of SARS is impossible. SARSis, however, the first severe <strong>and</strong> easily transmissible newdisease to emerge in the 21st century. 8 In the last century,major influenza A p<strong>and</strong>emics occurred in 1918, 1957, <strong>and</strong>1968, although with the possible exception of the 1918 experiencenone of these p<strong>and</strong>emics occurred in totally immunologicallyvirgin human populations. 2 In the mid-1950s,p<strong>and</strong>emic infection <strong>and</strong> disease caused by echovirus 9occurred. 6 Because echovirus 9 apparently was a new virusat that time, the entire world population was susceptible,<strong>and</strong> over a period of approximately 4 years everyone becameinfected. Fortunately, in that p<strong>and</strong>emic disease, mortality<strong>and</strong> residual morbidity were rare occurrences.At present, SARS is of major concern because it has ahigh case-fatality rate of approximately 4 percent, <strong>and</strong> theentire world population lacks immunity to the coronavirusthat is the causative agent.The incubation period of SARS is 2 to 10 days, with mostcases occurring 2 to 7 days after exposure. Most transmissionshave been person-to-person through respiratory secretions.Because the coronavirus also has been isolated fromfeces, fecal-oral or fecal-nasal routes of transmission seempossible as well. In addition, large clusters of cases have suggestedthe possibility of environmental contamination viasewage or ventilation systems. 8A cluster of more than 300 cases linked to a single buildingin an estate of high-rise apartment buildings in HongKong has been noted. Transmission occurred in this buildingnot only among persons living on the same floor but alsoamong persons living on different floors.Another interesting observation has been the finding of“super spreaders.” A super spreader is a patient who apparentlyhas infected a large number of persons. For example, alarge cluster of cases in health care workers in Singaporemay have been related to contact with a single patient withkidney disease <strong>and</strong> diabetes. Another possible superspreader in Guangdong, China, may have infected as manyas 100 other persons.As of April 2003, most of the reported cases in the WHOdatabase have occurred in adults, <strong>and</strong> a large number ofsecondary cases have occurred in health care workers. The
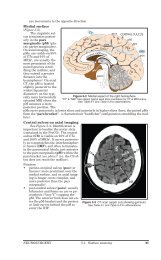
2390 SECTION XVII Viral Infectionsexperience of four of the authors from Hong Kong indicatesthat pediatric cases may play a significant role in the epidemiologyof SARS. However, because infection in youngchildren appears to be less severe, these cases may be overlooked,as they could blend in with other common respiratoryinfectious illnesses.Apparent death rates have varied considerably amonggeographic areas. These differences may relate to medicalcare issues, host factors, or perhaps the role of cofactors indisease. In Toronto, the death rate was particularly high,<strong>and</strong> here four of nine patients were found to be infected withboth the novel coronavirus <strong>and</strong> metapneumovirus. 12The progression of the present outbreak depends on severalfactors that affect the epidemic potential of specificinfectious agents <strong>and</strong> includes season, geography, climate,routes of transmission, infectious dose, <strong>and</strong> effectiveness ofpreventive measures.PathophysiologyFrom available studies involving adults <strong>and</strong> some children,the primary site of pathology clearly is the respiratory tract,with bilateral <strong>and</strong> patchy airspace consolidation in thelungs. 1, 11–13 In addition, viremia or toxemia is suggested bythe presence of lymphopenia <strong>and</strong> elevated serum enzymevalues (ALT, aspartate transaminase [AST], creatine phosphokinase[CPK], <strong>and</strong> lactate dehydrogenase [LDH]).Postmortem findings in two cases showed gross consolidationof the lungs <strong>and</strong> diffuse alveolar damage with pulmonaryedema <strong>and</strong> hyaline membrane formation. 11 Otherareas had cellular fibromyxoid organizing exudates in theairspaces. Interstitial spaces had only mild lymphocytic infiltrates.Vacuolated <strong>and</strong> multinucleated pneumocytes alsowere noted. In another study, similar findings were noted. 13Clinical PresentationThis analysis is based on observations of the first 10 pediatricpatients, 5 children <strong>and</strong> 5 adolescents, hospitalized in twoHong Kong hospitals in March 2003. All cases had historiesof contact: household contact in five, contact with health careworkers in three, <strong>and</strong> exposure to a widespread communityoutbreak in a densely populated residential estate in two.Three children <strong>and</strong> two adolescents had direct contact withindex adult family members with SARS. Five adult indexpatients had very severe SARS <strong>and</strong> required intensive care.Two patterns of illness were apparent. In adolescents, SARSis similar to the illness in adults. In contrast, the illness inchildren has a less severe presentation with no or minimalsystemic manifestations except fever, although evidence ofpneumonia is present on chest radiographs.multifocal airspace consolidations, <strong>and</strong> the features ofperipheral <strong>and</strong> alveolar opacities had the radiologic appearanceof bronchiolitis obliterans organizing pneumonia (Fig.<strong>189</strong>–3). All five patients had mild progressive consolidativechanges on serial chest radiographs, but complete resolutionoccurred within 14 days. The typical radiographic changes inone of the patients are illustrated in Figure <strong>189</strong>–4. None ofthe children had abnormal interstitial patterns, bronchialdilatation, pleural effusion, cavitation, or mediastinal lymphadenopathy.In spite of the radiographic findings, wheezing <strong>and</strong> inspiratoryrales were absent or not prominent on physical examination.None of the five patients developed evidence ofsevere respiratory distress throughout the course of their illnesses,<strong>and</strong> none was administered supplemental oxygen.The duration of fever was 3 to 7 days. The most impressivelaboratory finding was leukopenia with an absolute lymphopenia.The median lowest lymphocyte count was 1.1 ×10 9 /L. Lymphopenia occurred 3 to 4 days after the onset offever. Two children had modestly low platelet counts. LDHlevels were mildly elevated in all five children, but only onechild had an elevated CPK value <strong>and</strong> the ALT values werenormal in all five children.RT-PCR for the novel coronavirus was performed on anasopharyngeal aspirate specimen from one child <strong>and</strong>was positive. Thus, although all these children had appropriatecontact history <strong>and</strong> met the clinical case definition ofSARS, they possibly might not all have disease caused by thecoronavirus.ADOLESCENTSIn contrast with the children, all five of the adolescents presentedwith chills <strong>and</strong> rigor as well as fever. Four of the fivealso had cough, myalgia, <strong>and</strong> headache, <strong>and</strong> three hadcoryza. Other complaints included nausea in two patients<strong>and</strong> abdominal pain in one patient.Three patients had bilateral lower lobe opacifications onradiographs at the time of admission. These consolidativechanges progressed rapidly within days. Despite clinicalimprovement, the consolidative changes persisted into thesecond week of the illness. One patient had mild focal airspaceCHILDRENAll five children had fever <strong>and</strong> were febrile for 1 to 4 daysbefore hospital admission. Other features present on admissionincluded coryza in three patients <strong>and</strong> cough in fourpatients. One child had a febrile convulsion, <strong>and</strong> one childhad dizziness. None of the five children had sore throat,chills/rigor, myalgia, or headache.All children had evidence of pneumonia on initial chestradiographs. In four of the children, focal segmental consolidationswere seen on initial radiographs, <strong>and</strong> one childhad an ill-defined patchy consolidation. Computed tomography(CT) of the thorax performed on this patient showedFIGURE <strong>189</strong>–3 ■ One frame of a CT scan in a 2-year-old boy. TheCT study showed multiple areas of consolidation in the perihilar<strong>and</strong> subpleural regions <strong>and</strong> a ground glass opacification that wasprominent in the posterior aspects of the lungs.
CHAPTER <strong>189</strong> <strong>Coronaviruses</strong> <strong>and</strong> <strong>Toroviruses</strong>, <strong>Including</strong> <strong>Severe</strong> <strong>Acute</strong> Respiratory Syndrome (SARS) 2391ABFIGURE <strong>189</strong>–4 ■ Serial chest radiographs in a 7 1 / 2 -year-old boy who presented with fever <strong>and</strong> cough. A, Radiograph at presentationshows an ill-defined airspace consolidation in the periphery of the right upper lobe that abuts the horizontal fissure. B, This findingwas followed by an increased consolidation in the right upper lung field on day 5. Complete resolution of the airspace consolidationoccurred by day 14 (not shown). (From Hon, K. L. E., Leung, C. W., Cheng, W. T. F., et al.: Clinical presentations <strong>and</strong> outcome ofsevere acute respiratory syndrome in children. Lancet 361:1701, 2003.)consolidation similar to that seen in the CT scan of the 2-yearoldboy shown in Figure <strong>189</strong>–3. Complete resolution occurredwithin 14 days in this adolescent. Another patient did nothave any radiographic changes at presentation, but highresolutionCT confirmed the presence of focal consolidation inthe right lower lobe. This patient did not have any signs ofairspace consolidation in the subsequent serial radiographs.The hematologic manifestations in the teenagers weremore marked than those in the children. The lowest totalleukocyte count varied between 1.7 <strong>and</strong> 4.7 × 10 9 /L, <strong>and</strong> thelymphocyte counts were between 0.3 <strong>and</strong> 0.8 × 10 9 /L. Two ofthe five had modestly low platelet counts. All adolescentshad mildly elevated ALT values, <strong>and</strong> four had mildly elevatedLDH values. Only one patient had a slightly elevatedCPK value. RT-PCR for the novel coronavirus was positivein three of five nasopharyngeal aspirate specimens. Four ofthe five teenagers developed respiratory distress <strong>and</strong> oxygendesaturation between days 4 <strong>and</strong> 7 after the onset of fever.Two patients required oxygen supplementation throughnasal cannula (2–3 L/min). One patient required bilevelpositive airway pressure (BiPAP) support for 5 days.Another patient required intubation <strong>and</strong> mechanical ventilatorysupport for 4 days. The highest oxygen requirementfor the latter two patients was 50 percent. All patientsrecovered.Differential DiagnosisThe differential diagnosis of SARS in children includes themany viral, bacterial, mycoplasmal, <strong>and</strong> chlamydial agentsthat cause acute febrile respiratory illnesses in children(see Chapters 23, 25, 26, 27, 161, 181, 182, 185, <strong>189</strong>, 194,<strong>and</strong> 196). Particularly important to consider are agentsthat cause acute febrile respiratory illnesses in both adults<strong>and</strong> children. These agents include influenza viruses A<strong>and</strong> B, several adenoviral types, Chlamydia pneumoniae,Chlamydia psittaci, Mycoplasma pneumoniae, <strong>and</strong> Legionellapneumophila.Specific DiagnosisFor surveillance purposes, both the WHO <strong>and</strong> the Centersfor Disease Control <strong>and</strong> Prevention (CDC) have developedcase definitions. 5, 14 The CDC definition of a suspected caseas of March 22, 2003, was as follows:Respiratory illness of unknown etiology with onset sinceFebruary 1, 2003, <strong>and</strong> the following criteria:• Measured temperature greater than 100.4° F (> 38.0° C)• One or more clinical findings of respiratory illness (e.g.,cough, shortness of breath, difficulty breathing, hypoxia,or radiographic findings of either pneumonia or acuterespiratory distress syndrome)• Travel within 10 days of onset of symptoms to an areawith suspected or documented community transmissionof SARS (Hong Kong Special Administrative Region <strong>and</strong>Guangdong Province, China; Hanoi, Vietnam; <strong>and</strong> Singapore)(excluding areas with secondary cases limited tohealth care workers or direct household contacts)or• Close contact (close contact is defined as having caredfor, having lived with, or having had direct contact withrespiratory secretions <strong>and</strong>/or body fluids of a patient suspectedof having SARS) within 10 days of onset of symptomswith either a person with a respiratory illness <strong>and</strong>travel to a SARS area or a person under investigation orsuspected of having SARSSuspected cases with either radiographic evidence ofpneumonia or respiratory distress syndrome or evidence ofunexplained respiratory distress syndrome by autopsy weredesignated “probable” cases by the WHO case definition.Definitive etiologic diagnosis can be established by theidentification of the coronavirus in respiratory specimens byRT-PCR or the isolation of the organism in tissue culture.The identification of infection also can be determined by4, 16demonstrating specific antibody in sera by IFA or ELISA.Most convincing is the demonstration of seroconversion bystudied paired acute-phase <strong>and</strong> convalescent-phase sera.
Pleaseify allage.2392 SECTION XVII Viral InfectionsSARS should be suspected strongly in a previously healthypatient who has pneumonia <strong>and</strong> marked lymphopenia.TreatmentAs of April 2003, three case series of adult patients withSARS in which treatment data are presented have been published.11–13 Two series are from Hong Kong, 11, 13 <strong>and</strong> one isfrom Canada. 12 In the two series from Hong Kong, allpatients were treated with broad-spectrum antibacterialagents, oseltamivir, ribavirin, <strong>and</strong> corticosteroids. In theseries from Canada, the patients received similar therapyexcept that they were not administered corticosteroids. The10 pediatric cases noted here all received treatment programssimilar to the programs of the adults in Hong Kongexcept that oseltamivir was not used. Intravenous cefotaxime(25–50 mg/kg/dose, every 6 or 8 hours), oral clarithromycin(15 mg/kg/day, to a maximum dose of 250 mg twice daily),<strong>and</strong> oral ribavirin (40 mg/kg/day, in 2 or 3 divided doses)were started if a clinical diagnosis of SARS was suspected onadmission. Oral prednisolone (0.5 mg/kg/day at one hospital<strong>and</strong> 2 mg/kg/day at the other hospital) was added if nodecrease in fever or improvement in the general well-beingof the patient had occurred within 48 hours. If the childwas admitted with moderately severe symptoms ofhigh swinging fever <strong>and</strong> marked malaise, intravenousribavirin (20 mg/kg/day, in 3 divided doses) <strong>and</strong> intravenoushydrocortisone (2 mg/kg/dose, every 6 hours) in addition toantibacterial therapy was administered immediately afteradmission. For patients with persistent fever <strong>and</strong> progressiveclinical or radiologic deterioration, pulse intravenousmethylprednisolone (10 to 20 mg/kg/dose) was administered.The decision to provide further pulsed treatment was basedon clinical response. Antibacterial agents were discontinued5 days after defervescence. Ribavirin was administered for1 to 2 weeks, <strong>and</strong> corticosteroid was tapered over the courseof 2 to 4 weeks, depending on clinical response <strong>and</strong> radiologicresolution.Legitimate concerns exist with regard to the use of corticosteroidsin the treatment of SARS. Corticosteroid treatmentincreases the severity of illness <strong>and</strong> rate of death in many primaryviral illnesses such as measles <strong>and</strong> varicella. As SARSappears to be a primary viral infection caused by a new coronavirus,one might expect that corticosteroids would increaseviral load early in infection. The indication for corticosteroiduse in severe infectious diseases is the need to decreaseinflammation caused by excessive cytokine responses by thehost; the rationale for the use of steroids for SARS patients inHong Kong has been the belief that the severe lung damage isthe result of a “cytokine storm” similar to that observed ininfluenza A subtype H5N1 infection. 7 However, the severelymphopenia commonly noted in SARS suggests that theinflammatory response already is suppressed. If the abovespeculation were correct, one would expect a worsening of diseasedue to the corticosteroid treatment. At present, no evidenceshows that worsening has occurred, but this may beexplained by an antiviral effect of ribavirin, which has beenadministered concurrently in all cases.Because all patients with SARS have pneumonia, onemight assume that ribavirin treatment would be more effectiveif administered by small particle aerosol generator, as thismethod has been recommended previously for the treatmentof respiratory syncytial viral infections in infants <strong>and</strong> youngchildren (see Chapters 25 <strong>and</strong> 185). However, this methodof administration was not considered in Hong Kong becausethe first major outbreak of SARS appeared to have beenaccentuated by the use of a nebulizer on a general ward. 11PrognosisThe overall prognosis in children with SARS appears to begood. However, a small number of children have requiredtime in the intensive care unit <strong>and</strong> mechanical ventilation.The extent of lung damage in recovered children is notknown.PreventionThe mainstay of prevention of SARS is effective hospitalinfection control practices <strong>and</strong> quarantine proceduresoutside hospitals. In retrospect, many secondary cases ofSARS in health care personnel were preventable. Unfortunately,the vast majority of hospitals today have not beendesigned to h<strong>and</strong>le epidemic diseases that are transmittedby the respiratory route. Patients with respiratory illnessesconsistent with SARS should be isolated at triage <strong>and</strong> placedin a negative air pressure room for treatment. BecauseSARS is spread by the respiratory route <strong>and</strong> becausethe nose, mouth, <strong>and</strong> eyes are sites for primary infection,health care workers should wear masks <strong>and</strong> goggles whiletending patients. Because fomites appear to be importantfactors in the transmission of other respiratory viruses,medical equipment should be restricted to patient roomsor adequately disinfected after patient contact. Finally, adequateh<strong>and</strong>washing before <strong>and</strong> after patient contact isessential.Throughout history, quarantines (restraining the movementof people to prevent the spread of infectious disease)have been used successfully to prevent the spread of infectiousdiseases. 3 In the 1930s <strong>and</strong> 1940s, quarantine measureswere used effectively in reducing exposure duringperiods of epidemic poliomyelitis. However, during the last50 years, quarantine has fallen into disfavor because of opinionsthat it is unworkable <strong>and</strong> ineffective, which have beenfostered by beliefs that modern medicine, civil rights, <strong>and</strong>technology have made quarantine obsolete. However, quarantinemeasures have been used appropriately, <strong>and</strong> shouldcontinue to be used, in the present SARS epidemic. Restrictionsrelating to travel clearly are appropriate, as in thequarantining of exposed symptomatic persons who havetraveled to disease-free geographic areas.REFERENCES1. Ahuja, A. T., <strong>and</strong> Wong, J. K. T.: Radiological appearances of recent casesof atypical pneumonia in Hong Kong. www.droid.cunk.edu.ki/we/atypical_pneumonia/atypical_pneumonia.htm, 2003.2. Boyer, K. M., <strong>and</strong> Cherry, J. D.: Influenza. In Feigin, R. D., Cherry, J. D.(eds.): Textbook of Pediatric Infectious Diseases. 1st ed. Philadelphia,W. B. Saunders, 1981, pp. 1298–1316.3. Bromberg, J. S., <strong>and</strong> Vidich, C.: Twenty-first century role for quarantines.Los Angeles Times, April 9, 2003; B13.4. Centers for Disease Control <strong>and</strong> Prevention: <strong>Severe</strong> acute respiratorysyndrome (SARS) <strong>and</strong> coronavirus testing—United States, 2003. M. M.W. R. Morb. Mortal. Wkly. Rep. 52(14):297–302, 2003.5. Centers for Disease Control <strong>and</strong> Prevention: Update: Outbreak of severeacute respiratory syndrome—worldwide, 2003. M. M. W. R. Morb. Mortal.Wkly. Rep. 52(15):332–336, 2003.6. Cherry, J. D.: Enteroviruses: Coxsackieviruses, echoviruses, <strong>and</strong>polioviruses. In Feigin, R. D., <strong>and</strong> Cherry, J. D. (eds.): Textbook of PediatricInfectious Diseases. 4th ed. Philadelphia, W. B. Saunders, 1998, pp.1787–1839.7. Cheung, C., Poon, L., Lau, A., et al.: Induction of proinflammatorycytokines in human macrophages by influenza A (H5N1) viruses: A mechanismfor the unusual severity of human disease? Lancet 360:1831–1837,2002.8. Heymann, D. L.: Status of the global SARS outbreak <strong>and</strong> lessons for theimmediate future. www.who.int/csr/sarsarchive/2003_04_11/en/print.html,2003.
CHAPTER 190 Hantaviruses 23939. Holmes, K. V.: <strong>Coronaviruses</strong>. In Knipe, D. M., <strong>and</strong> Howley, P. M. (eds.):Fields Virology. Vol 1. Philadelphia, Lippincott Williams & Wilkins, 2001,pp. 1187–1203.10. Lai, M. M. C., <strong>and</strong> Holmes, K. V.: Coronaviridae: The viruses <strong>and</strong> theirreplication. In Knipe, D. M., <strong>and</strong> Howley, P. M. (eds.): Fields Virology.Vol. 1. Philadelphia, Lippincott Williams & Wilkins, 2001, pp. 685–722.11. Lee, N., Hui, D., Wu, A., et al.: A major outbreak of severe acuterespiratory syndrome in Hong Kong. N. Engl. J. Med. www.nejm.org,2003.12. Poutanen, S. M., Low, D. E., Henry, B., et al.: Identification of severeacute respiratory syndrome in Canada. N. Engl. J. Med. www.nejm.org,2003.13. Tsang, K. W., Ho, P. L., Ooi, G. C., et al.: A cluster of cases of severe acuterespiratory syndrome in Hong Kong. N. Engl. J. Med. www.nejm.org, 2003.14. World Health Organization: Case definitions for surveillance of severeacute respiratory syndrome (SARS). www.who.int/csr/sars/casedefinition/en/print.html, April 1, 2003.15. World Health Organization: <strong>Severe</strong> acute respiratory syndrome (SARS):Multi-country outbreak—Update 24. www.who.int/csr/dom/2003_04_08/en/print.html, April 8, 2003.16. World Health Organization: Summary on major findings in relation to coronavirusby members of the WHO multi-centre collaborative network onSARS aetiology <strong>and</strong> diagnosis. www.who.int/csr/sars/findings/en/print.html,April 4, 2003.SUBSECTION 11BUNYAVIRIDAECHAPTER190 HantavirusesC. J. PETERS ■ LOUISA E. CHAPMAN ■ KELLY T. MCKEE, JR.Historical PerspectiveHantaviruses are the etiologic agents of a diverse group ofrodent-borne hemorrhagic fevers that are responsible forconsiderable morbidity <strong>and</strong> mortality worldwide. Thoughrecognized by so-called modern medicine only relativelyrecently, clinical syndromes now known to be associatedwith these viruses have been described by traditional practitionersacross the globe from antiquity. 11, 62, 76 In the 20thcentury, Soviet scientists reported sporadic outbreaks offebrile renal failure with hemorrhage in the eastern SovietUnion between 1913 <strong>and</strong> 1930, 8 <strong>and</strong> Japanese <strong>and</strong> Soviet scientistsrecognized annual outbreaks of a similar syndrome29, 75, 103, 109in Manchuria <strong>and</strong> Siberia between 1932 <strong>and</strong> 1935.In 1934, Swedish scientists described a novel disorder characterizedby fever, abdominal <strong>and</strong> back pain, <strong>and</strong> renalabnormalities 80, 124 ; epidemic disease with these featuresoccurred among German <strong>and</strong> Finnish troops stationed in44, 106Lapl<strong>and</strong> during World War II.North American medical practitioners initially becameacquainted with a similar syndrome in the early 1950s,when a previously unrecognized febrile illness characterizedby shock, hemorrhage, <strong>and</strong> renal failure developed in thous<strong>and</strong>sof soldiers serving with United Nation forces duringthe Korean War. Physicians called this syndrome, which hada mortality rate of 5 to 15 percent, epidemic hemorrhagic23, 77fever.In 1953, Gajdusek 28 noted similarities among epidemichemorrhagic fever, the severe <strong>and</strong> frequently fatal Far Easterndiseases described by Soviet <strong>and</strong> Japanese scientists, <strong>and</strong>the Sc<strong>and</strong>inavian disorder <strong>and</strong> proposed a common etiology.Subsequent validation of this hypothesis (see “The Organism”)ultimately prompted adoption of the collective termhemorrhagic fever with renal syndrome (HFRS) to describethis clinical entity characterized by varying degrees of renaldysfunction <strong>and</strong> hemorrhage with fever.In June 1993, investigation of a cluster of unexplainedrespiratory deaths in previously healthy young adult residentsof rural areas in the southwestern United States led torecognition of a “new” hemorrhagic fever in North America.Application of sophisticated serologic <strong>and</strong> virologic tools todiagnostic specimens from patients with this mysterious diseasequickly pointed to a hantavirus related to, but distinct57, 82from, those causing HFRS. This disorder, now calledhantavirus pulmonary syndrome (HPS), proved to be differentclinically from classic HFRS, but as virologic <strong>and</strong> epidemiologicstudies of the etiologic agent <strong>and</strong> its reservoirswere completed, similarities to other hantaviruses becameapparent.The OrganismCLASSIFICATION AND ANTIGENIC COMPOSITIONThe genus Hantavirus of the family Bunyaviridae wasdefined in 1985. 96, 98 The members of the Hantavirus genusare classified among the bunyaviruses because of their sharedmorphologic, physicochemical, <strong>and</strong> molecular properties.Like other members of the family Bunyaviridae, hantavirusesare negative-str<strong>and</strong>ed, lipid-enveloped RNA viruses withtripartite genomes; genomic segments are designated aslarge (L), medium (M), <strong>and</strong> small (S). Also like otherbunyaviruses, they display Golgi-associated morphogenesis<strong>and</strong> usually acquire their envelopes by budding into intracytoplasmicvacuoles. 99 However, they are serologically distinctfrom other family members <strong>and</strong> possess unique terminalgenomic sequences. 96 The Hantavirus genus contains theonly members of Bunyaviridae that lack arthropod vectors.Each hantavirus is highly adapted to a specific rodentspecies <strong>and</strong> depends on persistent asymptomatic infection inwild rodents for maintenance in nature.