Polyphenylene Nanostructures - Cluster for Molecular Chemistry
Polyphenylene Nanostructures - Cluster for Molecular Chemistry
Polyphenylene Nanostructures - Cluster for Molecular Chemistry
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
1756 Chemical Reviews, 1999, Vol. 99, No. 7 Berresheim et al.<br />
Scheme 14 Scheme 15<br />
substituents in the 2, 2′, 7, and 7′ positions to obtain<br />
70, Scheme 14. The compounds obtained were more<br />
soluble than the corresponding single-chain oligophenylenes.<br />
Furthermore, these spiro compounds<br />
were shown to be organic glasses with extremely high<br />
glass-transition temperatures and high thermal stability<br />
(Chapter VI.A). 127,129<br />
III. Routes To Branched Oligophenylenes<br />
A. The Intramolecular [4+2]-Cycloaddition and<br />
Subsequent Aromatization of Appropriate<br />
Phenylenevinylene Derivatives<br />
Even the step-ladder or ladder polymers 43, 96 45, 97<br />
50, 98 58, 115 and, in particular, the ribbon polymer<br />
54, 113 based on the 1-dimensional poly(para-phenylene)<br />
12, deviate from the topology of a singlestranded<br />
polymer through the bridging of neighboring<br />
para-phenylene units. In the following discussion,<br />
our attention will be on monodisperse, topologically<br />
defined oligophenylene derivatives in which the step<br />
from 1- to 2-dimensionality is complete.<br />
An elegant entry to this type of branched oligophenylene<br />
structures was obtained, <strong>for</strong> example, by<br />
the intramolecular [4+2]-cycloaddition of suitable<br />
phenylenvinylene derivatives, followed by aromatization<br />
of the resulting cyclohexene structures. 130 This<br />
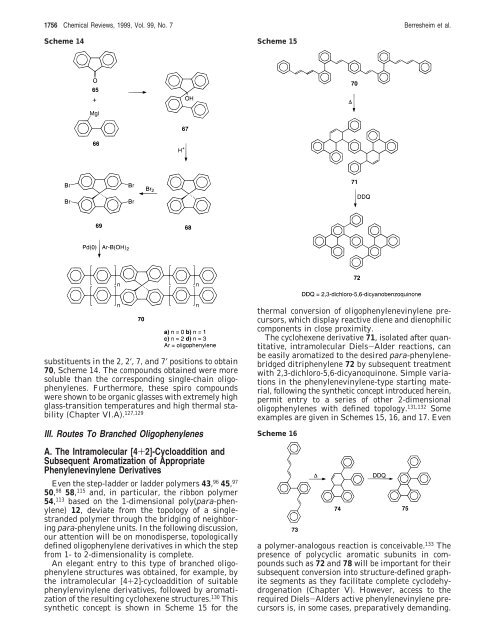
synthetic concept is shown in Scheme 15 <strong>for</strong> the<br />
thermal conversion of oligophenylenevinylene precursors,<br />
which display reactive diene and dienophilic<br />
components in close proximity.<br />
The cyclohexene derivative 71, isolated after quantitative,<br />
intramolecular Diels-Alder reactions, can<br />
be easily aromatized to the desired para-phenylenebridged<br />
ditriphenylene 72 by subsequent treatment<br />
with 2,3-dichloro-5,6-dicyanoquinone. Simple variations<br />
in the phenylenevinylene-type starting material,<br />
following the synthetic concept introduced herein,<br />
permit entry to a series of other 2-dimensional<br />
oligophenylenes with defined topology. 131,132 Some<br />
examples are given in Schemes 15, 16, and 17. Even<br />
Scheme 16<br />
a polymer-analogous reaction is conceivable. 133 The<br />
presence of polycyclic aromatic subunits in compounds<br />
such as 72 and 78 will be important <strong>for</strong> their<br />
subsequent conversion into structure-defined graphite<br />
segments as they facilitate complete cyclodehydrogenation<br />
(Chapter V). However, access to the<br />
required Diels-Alders active phenylenevinylene precursors<br />
is, in some cases, preparatively demanding.