Polyphenylene Nanostructures - Cluster for Molecular Chemistry
Polyphenylene Nanostructures - Cluster for Molecular Chemistry
Polyphenylene Nanostructures - Cluster for Molecular Chemistry
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
1752 Chemical Reviews, 1999, Vol. 99, No. 7 Berresheim et al.<br />
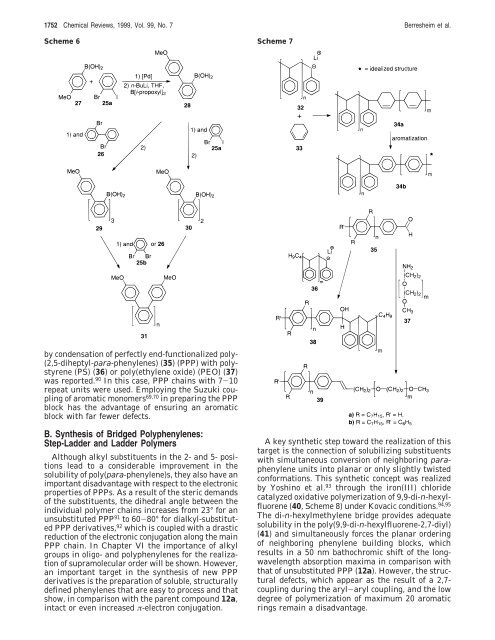
Scheme 6 Scheme 7<br />
by condensation of perfectly end-functionalized poly-<br />
(2,5-diheptyl-para-phenylenes) (35) (PPP) with polystyrene<br />
(PS) (36) or poly(ethylene oxide) (PEO) (37)<br />
was reported. 90 In this case, PPP chains with 7-10<br />
repeat units were used. Employing the Suzuki coupling<br />
of aromatic monomers 69,70 in preparing the PPP<br />
block has the advantage of ensuring an aromatic<br />
block with far fewer defects.<br />
B. Synthesis of Bridged <strong>Polyphenylene</strong>s:<br />
Step-Ladder and Ladder Polymers<br />
Although alkyl substituents in the 2- and 5- positions<br />
lead to a considerable improvement in the<br />
solubility of poly(para-phenylene)s, they also have an<br />
important disadvantage with respect to the electronic<br />
properties of PPPs. As a result of the steric demands<br />
of the substituents, the dihedral angle between the<br />
individual polymer chains increases from 23° <strong>for</strong> an<br />
unsubstituted PPP 91 to 60-80° <strong>for</strong> dialkyl-substituted<br />
PPP derivatives, 92 which is coupled with a drastic<br />
reduction of the electronic conjugation along the main<br />
PPP chain. In Chapter VI the importance of alkyl<br />
groups in oligo- and polyphenylenes <strong>for</strong> the realization<br />
of supramolecular order will be shown. However,<br />
an important target in the synthesis of new PPP<br />
derivatives is the preparation of soluble, structurally<br />
defined phenylenes that are easy to process and that<br />
show, in comparison with the parent compound 12a,<br />
intact or even increased π-electron conjugation.<br />
A key synthetic step toward the realization of this<br />
target is the connection of solubilizing substituents<br />
with simultaneous conversion of neighboring paraphenylene<br />
units into planar or only slightly twisted<br />
con<strong>for</strong>mations. This synthetic concept was realized<br />
by Yoshino et al. 93 through the iron(III) chloride<br />
catalyzed oxidative polymerization of 9,9-di-n-hexylfluorene<br />
(40, Scheme 8) under Kovacic conditions. 94,95<br />
The di-n-hexylmethylene bridge provides adequate<br />
solubility in the poly(9,9-di-n-hexylfluorene-2,7-diyl)<br />
(41) and simultaneously <strong>for</strong>ces the planar ordering<br />
of neighboring phenylene building blocks, which<br />
results in a 50 nm bathochromic shift of the longwavelength<br />
absorption maxima in comparison with<br />
that of unsubstituted PPP (12a). However, the structural<br />
defects, which appear as the result of a 2,7coupling<br />
during the aryl-aryl coupling, and the low<br />
degree of polymerization of maximum 20 aromatic<br />
rings remain a disadvantage.