Polyphenylene Nanostructures - Cluster for Molecular Chemistry
Polyphenylene Nanostructures - Cluster for Molecular Chemistry
Polyphenylene Nanostructures - Cluster for Molecular Chemistry
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
1770 Chemical Reviews, 1999, Vol. 99, No. 7 Berresheim et al.<br />
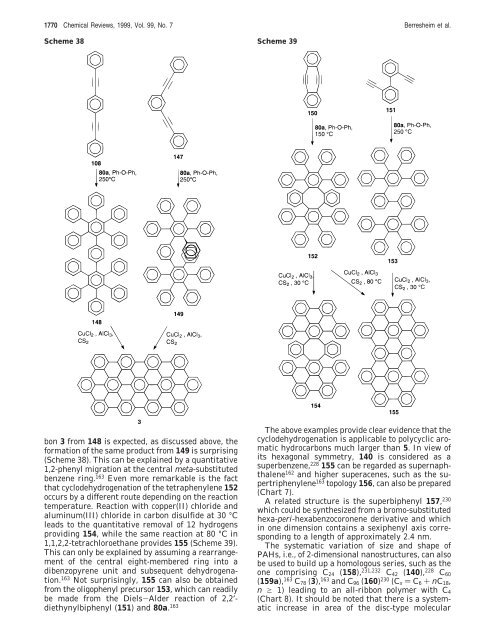
Scheme 38 Scheme 39<br />
bon 3 from 148 is expected, as discussed above, the<br />
<strong>for</strong>mation of the same product from 149 is surprising<br />
(Scheme 38). This can be explained by a quantitative<br />
1,2-phenyl migration at the central meta-substituted<br />
benzene ring. 163 Even more remarkable is the fact<br />
that cyclodehydrogenation of the tetraphenylene 152<br />
occurs by a different route depending on the reaction<br />
temperature. Reaction with copper(II) chloride and<br />
aluminum(III) chloride in carbon disulfide at 30 °C<br />
leads to the quantitative removal of 12 hydrogens<br />
providing 154, while the same reaction at 80 °C in<br />
1,1,2,2-tetrachloroethane provides 155 (Scheme 39).<br />
This can only be explained by assuming a rearrangement<br />
of the central eight-membered ring into a<br />
dibenzopyrene unit and subsequent dehydrogenation.<br />
163 Not surprisingly, 155 can also be obtained<br />
from the oligophenyl precursor 153, which can readily<br />
be made from the Diels-Alder reaction of 2,2′diethynylbiphenyl<br />
(151) and 80a. 163<br />
The above examples provide clear evidence that the<br />
cyclodehydrogenation is applicable to polycyclic aromatic<br />
hydrocarbons much larger than 5. In view of<br />
its hexagonal symmetry, 140 is considered as a<br />
superbenzene, 228 155 can be regarded as supernaphthalene<br />
162 and higher superacenes, such as the supertriphenylene<br />
163 topology 156, can also be prepared<br />
(Chart 7).<br />
A related structure is the superbiphenyl 157, 230<br />
which could be synthesized from a bromo-substituted<br />
hexa-peri-hexabenzocoronene derivative and which<br />
in one dimension contains a sexiphenyl axis corresponding<br />
to a length of approximately 2.4 nm.<br />
The systematic variation of size and shape of<br />
PAHs, i.e., of 2-dimensional nanostructures, can also<br />
be used to build up a homologous series, such as the<br />
one comprising C24 (158), 231,232 C42 (140), 228 C60<br />
(159a), 163 C78 (3), 163 and C96 (160) 230 (Cx ) C6 + nC18,<br />
n g 1) leading to an all-ribbon polymer with C4<br />
(Chart 8). It should be noted that there is a systematic<br />
increase in area of the disc-type molecular