Polyphenylene Nanostructures - Cluster for Molecular Chemistry
Polyphenylene Nanostructures - Cluster for Molecular Chemistry
Polyphenylene Nanostructures - Cluster for Molecular Chemistry
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
1764 Chemical Reviews, 1999, Vol. 99, No. 7 Berresheim et al.<br />
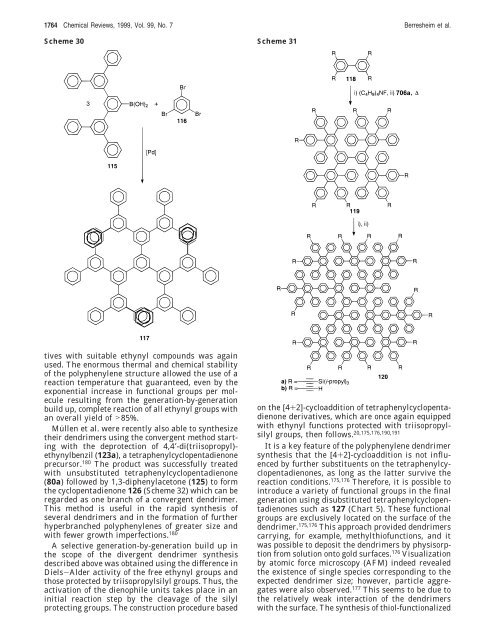
Scheme 30 Scheme 31<br />
tives with suitable ethynyl compounds was again<br />
used. The enormous thermal and chemical stability<br />
of the polyphenylene structure allowed the use of a<br />
reaction temperature that guaranteed, even by the<br />
exponential increase in functional groups per molecule<br />
resulting from the generation-by-generation<br />
build up, complete reaction of all ethynyl groups with<br />
an overall yield of >85%.<br />
Müllen et al. were recently also able to synthesize<br />
their dendrimers using the convergent method starting<br />
with the deprotection of 4,4′-di(triisopropyl)ethynylbenzil<br />
(123a), a tetraphenylcyclopentadienone<br />
precursor. 180 The product was successfully treated<br />
with unsubstituted tetraphenylcyclopentadienone<br />
(80a) followed by 1,3-diphenylacetone (125) to <strong>for</strong>m<br />
the cyclopentadienone 126 (Scheme 32) which can be<br />
regarded as one branch of a convergent dendrimer.<br />
This method is useful in the rapid synthesis of<br />
several dendrimers and in the <strong>for</strong>mation of further<br />
hyperbranched polyphenylenes of greater size and<br />
with fewer growth imperfections. 180<br />
A selective generation-by-generation build up in<br />
the scope of the divergent dendrimer synthesis<br />
described above was obtained using the difference in<br />
Diels-Alder activity of the free ethynyl groups and<br />
those protected by triisopropylsilyl groups. Thus, the<br />
activation of the dienophile units takes place in an<br />
initial reaction step by the cleavage of the silyl<br />
protecting groups. The construction procedure based<br />
706a<br />
on the [4+2]-cycloaddition of tetraphenylcyclopentadienone<br />
derivatives, which are once again equipped<br />
with ethynyl functions protected with triisopropylsilyl<br />
groups, then follows. 20,175,176,190,191<br />
It is a key feature of the polyphenylene dendrimer<br />
synthesis that the [4+2]-cycloaddition is not influenced<br />
by further substituents on the tetraphenylcyclopentadienones,<br />
as long as the latter survive the<br />
reaction conditions. 175,176 There<strong>for</strong>e, it is possible to<br />
introduce a variety of functional groups in the final<br />
generation using disubstituted tetraphenylcyclopentadienones<br />
such as 127 (Chart 5). These functional<br />
groups are exclusively located on the surface of the<br />
dendrimer. 175,176 This approach provided dendrimers<br />
carrying, <strong>for</strong> example, methylthiofunctions, and it<br />
was possible to deposit the dendrimers by physisorption<br />
from solution onto gold surfaces. 176 Visualization<br />
by atomic <strong>for</strong>ce microscopy (AFM) indeed revealed<br />
the existence of single species corresponding to the<br />
expected dendrimer size; however, particle aggregates<br />
were also observed. 177 This seems to be due to<br />
the relatively weak interaction of the dendrimers<br />
with the surface. The synthesis of thiol-functionalized