Polyphenylene Nanostructures - Cluster for Molecular Chemistry
Polyphenylene Nanostructures - Cluster for Molecular Chemistry
Polyphenylene Nanostructures - Cluster for Molecular Chemistry
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
1766 Chemical Reviews, 1999, Vol. 99, No. 7 Berresheim et al.<br />
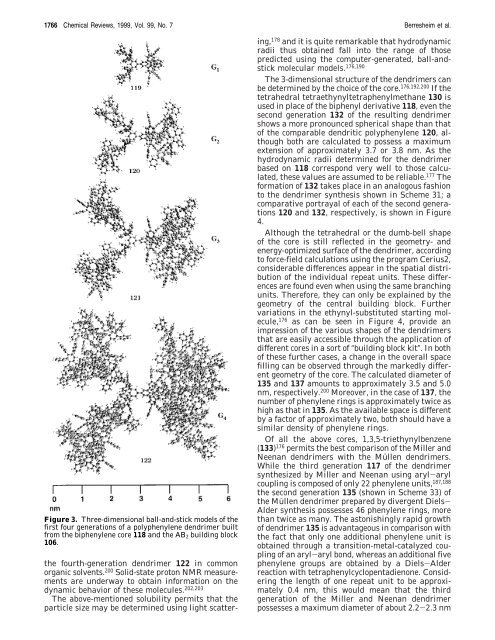
Figure 3. Three-dimensional ball-and-stick models of the<br />
first four generations of a polyphenylene dendrimer built<br />
from the biphenylene core 118 and the AB2 building block<br />
106.<br />
the fourth-generation dendrimer 122 in common<br />
organic solvents. 200 Solid-state proton NMR measurements<br />
are underway to obtain in<strong>for</strong>mation on the<br />
dynamic behavior of these molecules. 202,203<br />
The above-mentioned solubility permits that the<br />
particle size may be determined using light scatter-<br />
ing, 178 and it is quite remarkable that hydrodynamic<br />
radii thus obtained fall into the range of those<br />
predicted using the computer-generated, ball-andstick<br />
molecular models. 176,190<br />
The 3-dimensional structure of the dendrimers can<br />
be determined by the choice of the core. 176,192,200 If the<br />
tetrahedral tetraethynyltetraphenylmethane 130 is<br />
used in place of the biphenyl derivative 118, even the<br />
second generation 132 of the resulting dendrimer<br />
shows a more pronounced spherical shape than that<br />
of the comparable dendritic polyphenylene 120, although<br />
both are calculated to possess a maximum<br />
extension of approximately 3.7 or 3.8 nm. As the<br />
hydrodynamic radii determined <strong>for</strong> the dendrimer<br />
based on 118 correspond very well to those calculated,<br />
these values are assumed to be reliable. 177 The<br />
<strong>for</strong>mation of 132 takes place in an analogous fashion<br />
to the dendrimer synthesis shown in Scheme 31; a<br />
comparative portrayal of each of the second generations<br />
120 and 132, respectively, is shown in Figure<br />
4.<br />
Although the tetrahedral or the dumb-bell shape<br />
of the core is still reflected in the geometry- and<br />
energy-optimized surface of the dendrimer, according<br />
to <strong>for</strong>ce-field calculations using the program Cerius2,<br />
considerable differences appear in the spatial distribution<br />
of the individual repeat units. These differences<br />
are found even when using the same branching<br />
units. There<strong>for</strong>e, they can only be explained by the<br />
geometry of the central building block. Further<br />
variations in the ethynyl-substituted starting molecule,<br />
176 as can be seen in Figure 4, provide an<br />
impression of the various shapes of the dendrimers<br />
that are easily accessible through the application of<br />
different cores in a sort of “building block kit”. In both<br />
of these further cases, a change in the overall space<br />
filling can be observed through the markedly different<br />
geometry of the core. The calculated diameter of<br />
135 and 137 amounts to approximately 3.5 and 5.0<br />
nm, respectively. 200 Moreover, in the case of 137, the<br />
number of phenylene rings is approximately twice as<br />
high as that in 135. As the available space is different<br />
by a factor of approximately two, both should have a<br />
similar density of phenylene rings.<br />
Of all the above cores, 1,3,5-triethynylbenzene<br />
(133) 176 permits the best comparison of the Miller and<br />
Neenan dendrimers with the Müllen dendrimers.<br />
While the third generation 117 of the dendrimer<br />
synthesized by Miller and Neenan using aryl-aryl<br />
coupling is composed of only 22 phenylene units, 187,188<br />
the second generation 135 (shown in Scheme 33) of<br />
the Müllen dendrimer prepared by divergent Diels-<br />
Alder synthesis possesses 46 phenylene rings, more<br />
than twice as many. The astonishingly rapid growth<br />
of dendrimer 135 is advantageous in comparison with<br />
the fact that only one additional phenylene unit is<br />
obtained through a transition-metal-catalyzed coupling<br />
of an aryl-aryl bond, whereas an additional five<br />
phenylene groups are obtained by a Diels-Alder<br />
reaction with tetraphenylcyclopentadienone. Considering<br />
the length of one repeat unit to be approximately<br />
0.4 nm, this would mean that the third<br />
generation of the Miller and Neenan dendrimer<br />
possesses a maximum diameter of about 2.2-2.3 nm