3.091 â Introduction to Solid State Chemistry Lecture Notes No. 2 ...
3.091 â Introduction to Solid State Chemistry Lecture Notes No. 2 ...
3.091 â Introduction to Solid State Chemistry Lecture Notes No. 2 ...
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
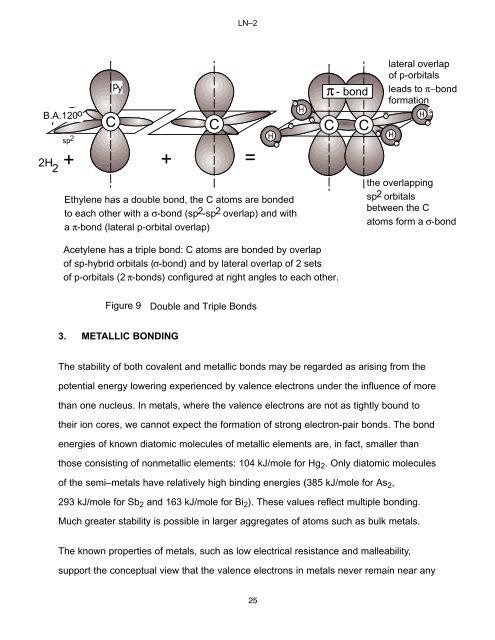
LN–2B.A.120osp 22H 2+pyπ - bondC C -C C+ =Ethylene has a double bond, the C a<strong>to</strong>ms are bonded<strong>to</strong> each other with a σ-bond (sp2-sp2 overlap) and witha π-bond (lateral p-orbital overlap)H--H-Acetylene has a triple bond: C a<strong>to</strong>ms are bonded by overlapof sp-hybrid orbitals (σ-bond) and by lateral overlap of 2 setsof p-orbitals (2 π-bonds) configured at right angles <strong>to</strong> each other.-lateral overlapof p-orbitalsleads <strong>to</strong> π−bondformationsH-H-the overlappingsp2 orbitalsbetween the Ca<strong>to</strong>ms form a σ-bond-Figure 9Double and Triple Bonds3. METALLIC BONDINGThe stability of both covalent and metallic bonds may be regarded as arising from thepotential energy lowering experienced by valence electrons under the influence of morethan one nucleus. In metals, where the valence electrons are not as tightly bound <strong>to</strong>their ion cores, we cannot expect the formation of strong electron-pair bonds. The bondenergies of known dia<strong>to</strong>mic molecules of metallic elements are, in fact, smaller thanthose consisting of nonmetallic elements: 104 kJ/mole for Hg 2 . Only dia<strong>to</strong>mic moleculesof the semi–metals have relatively high binding energies (385 kJ/mole for As 2 ,293 kJ/mole for Sb 2 and 163 kJ/mole for Bi 2 ). These values reflect multiple bonding.Much greater stability is possible in larger aggregates of a<strong>to</strong>ms such as bulk metals.The known properties of metals, such as low electrical resistance and malleability,support the conceptual view that the valence electrons in metals never remain near any25


![18.03 Class 21, April 3 Fun with Fourier series [1] If f(t) is any decent ...](https://img.yumpu.com/51148985/1/190x245/1803-class-21-april-3-fun-with-fourier-series-1-if-ft-is-any-decent-.jpg?quality=85)












