3.091 â Introduction to Solid State Chemistry Lecture Notes No. 2 ...
3.091 â Introduction to Solid State Chemistry Lecture Notes No. 2 ...
3.091 â Introduction to Solid State Chemistry Lecture Notes No. 2 ...
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
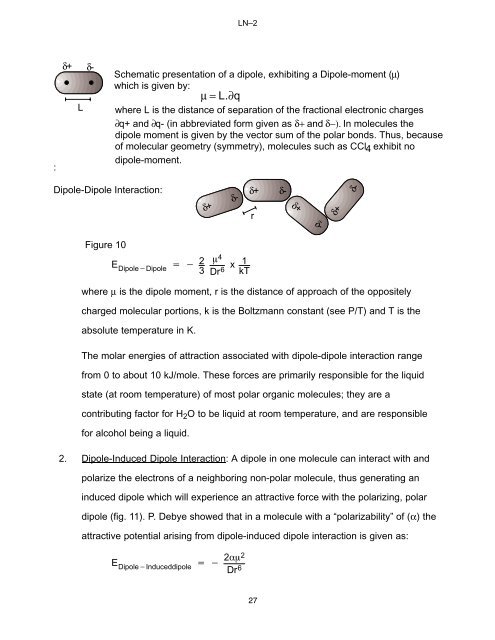
LN–2:δ+ δ-LSchematic presentation of a dipole, exhibiting a Dipole-moment (µ)which is given by:µ= L.∂qwhere L is the distance of separation of the fractional electronic charges∂q+ and ∂q- (in abbreviated form given as δ+ and δ−). In molecules thedipole moment is given by the vec<strong>to</strong>r sum of the polar bonds. Thus, becauseof molecular geometry (symmetry), molecules such as CCl4 exhibit nodipole-moment.Dipole-Dipole Interaction: δ+ δ-δ+ δ-rδ+ δ-δ+ δ-Figure 102 m4 E +* x 1Dipole*Dipole 3 Dr 6 kTwhere µ is the dipole moment, r is the distance of approach of the oppositelycharged molecular portions, k is the Boltzmann constant (see P/T) and T is theabsolute temperature in K.The molar energies of attraction associated with dipole-dipole interaction rangefrom 0 <strong>to</strong> about 10 kJ/mole. These forces are primarily responsible for the liquidstate (at room temperature) of most polar organic molecules; they are acontributing fac<strong>to</strong>r for H 2 O <strong>to</strong> be liquid at room temperature, and are responsiblefor alcohol being a liquid.2. Dipole-Induced Dipole Interaction: A dipole in one molecule can interact with andpolarize the electrons of a neighboring non-polar molecule, thus generating aninduced dipole which will experience an attractive force with the polarizing, polardipole (fig. 11). P. Debye showed that in a molecule with a “polarizability” of (α) theattractive potential arising from dipole-induced dipole interaction is given as:2am 2E Dipole*Induceddipole+*Dr 6 27


![18.03 Class 21, April 3 Fun with Fourier series [1] If f(t) is any decent ...](https://img.yumpu.com/51148985/1/190x245/1803-class-21-april-3-fun-with-fourier-series-1-if-ft-is-any-decent-.jpg?quality=85)












