Thermal Analysis of Polymers - Mettler Toledo
Thermal Analysis of Polymers - Mettler Toledo
Thermal Analysis of Polymers - Mettler Toledo
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
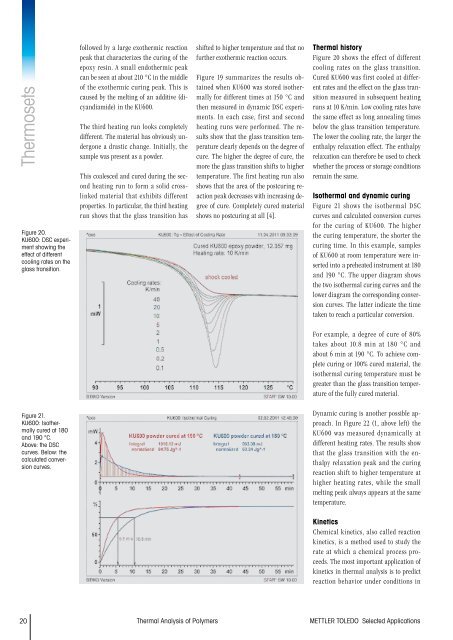
ThermosetsFigure 20.KU600: DSC experimentshowing theeffect <strong>of</strong> differentcooling rates on theglass transition.followed by a large exothermic reactionpeak that characterizes the curing <strong>of</strong> theepoxy resin. A small endothermic peakcan be seen at about 210 °C in the middle<strong>of</strong> the exothermic curing peak. This iscaused by the melting <strong>of</strong> an additive (dicyandiamide)in the KU600.The third heating run looks completelydifferent. The material has obviously undergonea drastic change. Initially, thesample was present as a powder.This coalesced and cured during the secondheating run to form a solid crosslinkedmaterial that exhibits differentproperties. In particular, the third heatingrun shows that the glass transition hasshifted to higher temperature and that n<strong>of</strong>urther exothermic reaction occurs.Figure 19 summarizes the results obtainedwhen KU600 was stored isothermallyfor different times at 150 °C andthen measured in dynamic DSC experiments.In each case, first and secondheating runs were performed. The resultsshow that the glass transition temperatureclearly depends on the degree <strong>of</strong>cure. The higher the degree <strong>of</strong> cure, themore the glass transition shifts to highertemperature. The first heating run alsoshows that the area <strong>of</strong> the postcuring reactionpeak decreases with increasing degree<strong>of</strong> cure. Completely cured materialshows no postcuring at all [4].<strong>Thermal</strong> historyFigure 20 shows the effect <strong>of</strong> differentcooling rates on the glass transition.Cured KU600 was first cooled at differentrates and the effect on the glass transitionmeasured in subsequent heatingruns at 10 K/min. Low cooling rates havethe same effect as long annealing timesbelow the glass transition temperature.The lower the cooling rate, the larger theenthalpy relaxation effect. The enthalpyrelaxation can therefore be used to checkwhether the process or storage conditionsremain the same.Isothermal and dynamic curingFigure 21 shows the isothermal DSCcurves and calculated conversion curvesfor the curing <strong>of</strong> KU600. The higherthe curing temperature, the shorter thecuring time. In this example, samples<strong>of</strong> KU600 at room temperature were insertedinto a preheated instrument at 180and 190 °C. The upper diagram showsthe two isothermal curing curves and thelower diagram the corresponding conversioncurves. The latter indicate the timetaken to reach a particular conversion.For example, a degree <strong>of</strong> cure <strong>of</strong> 80%takes about 10.8 min at 180 °C andabout 6 min at 190 °C. To achieve completecuring or 100% cured material, theisothermal curing temperature must begreater than the glass transition temperature<strong>of</strong> the fully cured material.Figure 21.KU600: Isothermallycured at 180and 190 °C.Above: the DSCcurves. Below: thecalculated conversioncurves.Dynamic curing is another possible approach.In Figure 22 (1, above left) theKU600 was measured dynamically atdifferent heating rates. The results showthat the glass transition with the enthalpyrelaxation peak and the curingreaction shift to higher temperature athigher heating rates, while the smallmelting peak always appears at the sametemperature.KineticsChemical kinetics, also called reactionkinetics, is a method used to study therate at which a chemical process proceeds.The most important application <strong>of</strong>kinetics in thermal analysis is to predictreaction behavior under conditions in20 <strong>Thermal</strong> <strong>Analysis</strong> <strong>of</strong> <strong>Polymers</strong> METTLER TOLEDO Selected Applications