Thermal Analysis of Polymers - Mettler Toledo
Thermal Analysis of Polymers - Mettler Toledo
Thermal Analysis of Polymers - Mettler Toledo
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
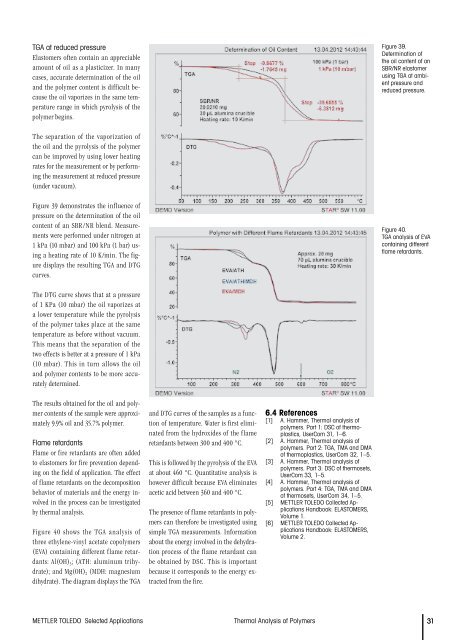
TGA at reduced pressureElastomers <strong>of</strong>ten contain an appreciableamount <strong>of</strong> oil as a plasticizer. In manycases, accurate determination <strong>of</strong> the oiland the polymer content is difficult becausethe oil vaporizes in the same temperaturerange in which pyrolysis <strong>of</strong> thepolymer begins.Figure 39.Determination <strong>of</strong>the oil content <strong>of</strong> anSBR/NR elastomerusing TGA at ambientpressure andreduced pressure.The separation <strong>of</strong> the vaporization <strong>of</strong>the oil and the pyrolysis <strong>of</strong> the polymercan be improved by using lower heatingrates for the measurement or by performingthe measurement at reduced pressure(under vacuum).Figure 39 demonstrates the influence <strong>of</strong>pressure on the determination <strong>of</strong> the oilcontent <strong>of</strong> an SBR/NR blend. Measurementswere performed under nitrogen at1 kPa (10 mbar) and 100 kPa (1 bar) usinga heating rate <strong>of</strong> 10 K/min. The figuredisplays the resulting TGA and DTGcurves.Figure 40.TGA analysis <strong>of</strong> EVAcontaining differentflame retardants.The DTG curve shows that at a pressure<strong>of</strong> 1 KPa (10 mbar) the oil vaporizes ata lower temperature while the pyrolysis<strong>of</strong> the polymer takes place at the sametemperature as before without vacuum.This means that the separation <strong>of</strong> thetwo effects is better at a pressure <strong>of</strong> 1 kPa(10 mbar). This in turn allows the oiland polymer contents to be more accuratelydetermined.The results obtained for the oil and polymercontents <strong>of</strong> the sample were approximately9.9% oil and 35.7% polymer.Flame retardantsFlame or fire retardants are <strong>of</strong>ten addedto elastomers for fire prevention dependingon the field <strong>of</strong> application. The effect<strong>of</strong> flame retardants on the decompositionbehavior <strong>of</strong> materials and the energy involvedin the process can be investigatedby thermal analysis.Figure 40 shows the TGA analysis <strong>of</strong>three ethylene-vinyl acetate copolymers(EVA) containing different flame retardants:Al(OH) 3 ; (ATH: aluminum trihydrate);and Mg(OH) 2 (MDH: magnesiumdihydrate). The diagram displays the TGAand DTG curves <strong>of</strong> the samples as a function<strong>of</strong> temperature. Water is first eliminatedfrom the hydroxides <strong>of</strong> the flameretardants between 300 and 400 °C.This is followed by the pyrolysis <strong>of</strong> the EVAat about 460 °C. Quantitative analysis ishowever difficult because EVA eliminatesacetic acid between 360 and 400 °C.The presence <strong>of</strong> flame retardants in polymerscan therefore be investigated usingsimple TGA measurements. Informationabout the energy involved in the dehydrationprocess <strong>of</strong> the flame retardant canbe obtained by DSC. This is importantbecause it corresponds to the energy extractedfrom the fire.6.4 References[1] A. Hammer, <strong>Thermal</strong> analysis <strong>of</strong>polymers. Part 1: DSC <strong>of</strong> thermoplastics,UserCom 31, 1–6.[2] A. Hammer, <strong>Thermal</strong> analysis <strong>of</strong>polymers. Part 2: TGA, TMA and DMA<strong>of</strong> thermoplastics, UserCom 32, 1–5.[3] A. Hammer, <strong>Thermal</strong> analysis <strong>of</strong>polymers. Part 3: DSC <strong>of</strong> thermosets,UserCom 33, 1–5.[4] A. Hammer, <strong>Thermal</strong> analysis <strong>of</strong>polymers. Part 4: TGA, TMA and DMA<strong>of</strong> thermosets, UserCom 34, 1–5.[5] METTLER TOLEDO Collected ApplicationsHandbook: ELASTOMERS,Volume 1.[6] METTLER TOLEDO Collected ApplicationsHandbook: ELASTOMERS,Volume 2.METTLER TOLEDO Selected Applications <strong>Thermal</strong> <strong>Analysis</strong> <strong>of</strong> <strong>Polymers</strong> 31