Yersinia Virulence Depends on Mimicry of Host Rho ... - ResearchGate
Yersinia Virulence Depends on Mimicry of Host Rho ... - ResearchGate
Yersinia Virulence Depends on Mimicry of Host Rho ... - ResearchGate
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
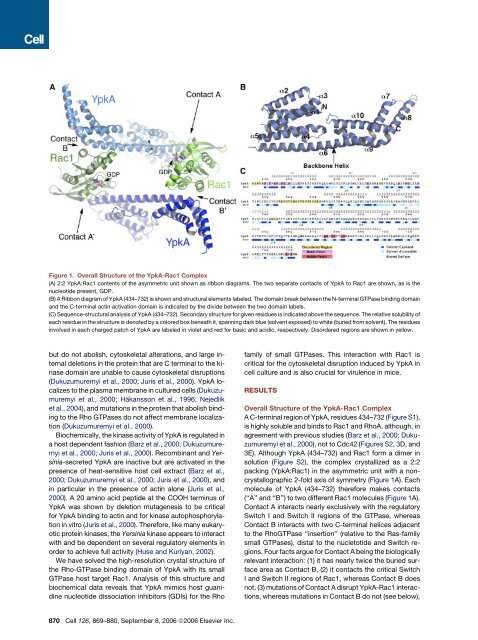
Figure 1. Overall Structure <strong>of</strong> the YpkA-Rac1 Complex(A) 2:2 YpkA:Rac1 c<strong>on</strong>tents <strong>of</strong> the asymmetric unit shown as ribb<strong>on</strong> diagrams. The two separate c<strong>on</strong>tacts <strong>of</strong> YpkA to Rac1 are shown, as is thenucleotide present, GDP.(B) A Ribb<strong>on</strong> diagram <strong>of</strong> YpkA (434–732) is shown and structural elements labeled. The domain break between the N-terminal GTPase binding domainand the C-terminal actin activati<strong>on</strong> domain is indicated by the divide between the two domain labels.(C) Sequence-structural analysis <strong>of</strong> YpkA (434–732). Sec<strong>on</strong>dary structure for given residues is indicated above the sequence. The relative solubility <strong>of</strong>each residue in the structure is denoted by a colored box beneath it, spanning dark blue (solvent exposed) to white (buried from solvent). The residuesinvolved in each charged patch <strong>of</strong> YpkA are labeled in violet and red for basic and acidic, respectively. Disordered regi<strong>on</strong>s are shown in yellow.but do not abolish, cytoskeletal alterati<strong>on</strong>s, and large internaldeleti<strong>on</strong>s in the protein that are C terminal to the kinasedomain are unable to cause cytoskeletal disrupti<strong>on</strong>s(Dukuzumuremyi et al., 2000; Juris et al., 2000). YpkA localizesto the plasma membrane in cultured cells (Dukuzumuremyiet al., 2000; Häkanss<strong>on</strong> et al., 1996; Nejedliket al., 2004), and mutati<strong>on</strong>s in the protein that abolish bindingto the <strong>Rho</strong> GTPases do not affect membrane localizati<strong>on</strong>(Dukuzumuremyi et al., 2000).Biochemically, the kinase activity <strong>of</strong> YpkA is regulated ina host dependent fashi<strong>on</strong> (Barz et al., 2000; Dukuzumuremyiet al., 2000; Juris et al., 2000). Recombinant and <str<strong>on</strong>g>Yersinia</str<strong>on</strong>g>-secretedYpkA are inactive but are activated in thepresence <strong>of</strong> heat-sensitive host cell extract (Barz et al.,2000; Dukuzumuremyi et al., 2000; Juris et al., 2000), andin particular in the presence <strong>of</strong> actin al<strong>on</strong>e (Juris et al.,2000). A 20 amino acid peptide at the COOH terminus <strong>of</strong>YpkA was shown by deleti<strong>on</strong> mutagenesis to be criticalfor YpkA binding to actin and for kinase autophosphorylati<strong>on</strong>in vitro (Juris et al., 2000). Therefore, like many eukaryoticprotein kinases, the <str<strong>on</strong>g>Yersinia</str<strong>on</strong>g> kinase appears to interactwith and be dependent <strong>on</strong> several regulatory elements inorder to achieve full activity (Huse and Kuriyan, 2002).We have solved the high-resoluti<strong>on</strong> crystal structure <strong>of</strong>the <strong>Rho</strong>-GTPase binding domain <strong>of</strong> YpkA with its smallGTPase host target Rac1. Analysis <strong>of</strong> this structure andbiochemical data reveals that YpkA mimics host guanidinenucleotide dissociati<strong>on</strong> inhibitors (GDIs) for the Rh<strong>of</strong>amily <strong>of</strong> small GTPases. This interacti<strong>on</strong> with Rac1 iscritical for the cytoskeletal disrupti<strong>on</strong> induced by YpkA incell culture and is also crucial for virulence in mice.RESULTSOverall Structure <strong>of</strong> the YpkA-Rac1 ComplexA C-terminal regi<strong>on</strong> <strong>of</strong> YpkA, residues 434–732 (Figure S1),is highly soluble and binds to Rac1 and <strong>Rho</strong>A, although, inagreement with previous studies (Barz et al., 2000; Dukuzumuremyiet al., 2000), not to Cdc42 (Figures S2, 3D, and3E). Although YpkA (434–732) and Rac1 form a dimer insoluti<strong>on</strong> (Figure S2), the complex crystallized as a 2:2packing (YpkA:Rac1) in the asymmetric unit with a n<strong>on</strong>crystallographic2-fold axis <strong>of</strong> symmetry (Figure 1A). Eachmolecule <strong>of</strong> YpkA (434–732) therefore makes c<strong>on</strong>tacts(‘‘A’’ and ‘‘B’’) to two different Rac1 molecules (Figure 1A).C<strong>on</strong>tact A interacts nearly exclusively with the regulatorySwitch I and Switch II regi<strong>on</strong>s <strong>of</strong> the GTPase, whereasC<strong>on</strong>tact B interacts with two C-terminal helices adjacentto the <strong>Rho</strong>GTPase ‘‘inserti<strong>on</strong>’’ (relative to the Ras-familysmall GTPases), distal to the nucletotide and Switch regi<strong>on</strong>s.Four facts argue for C<strong>on</strong>tact A being the biologicallyrelevant interacti<strong>on</strong>: (1) it has nearly twice the buried surfacearea as C<strong>on</strong>tact B, (2) it c<strong>on</strong>tacts the critical SwitchI and Switch II regi<strong>on</strong>s <strong>of</strong> Rac1, whereas C<strong>on</strong>tact B doesnot, (3) mutati<strong>on</strong>s <strong>of</strong> C<strong>on</strong>tact A disrupt YpkA-Rac1 interacti<strong>on</strong>s,whereas mutati<strong>on</strong>s in C<strong>on</strong>tact B do not (see below),870 Cell 126, 869–880, September 8, 2006 ª2006 Elsevier Inc.