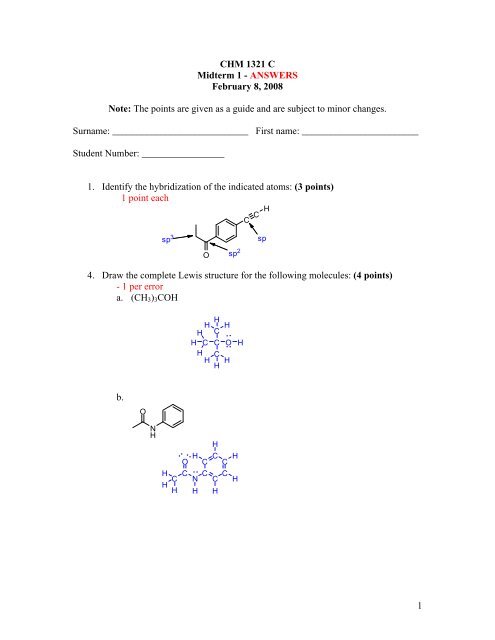
1 CHM 1321 C Midterm 1 - Université d'Ottawa
1 CHM 1321 C Midterm 1 - Université d'Ottawa
1 CHM 1321 C Midterm 1 - Université d'Ottawa
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Land/LotsPage 3 of 3MapArea Size LocationAskingPriceCommentsFALL 20115 50,038± s.f. 163 Pierce St. $2,700,000 EXCELLENT CORNER LOCATION;situated on I-280 off-ramp at signalizedintersection; fully entitled for 14,000 s.f.retail store and 1,400 kiosk; othercommercial uses also possible.Contact:Cassidy Turley/BT Commercial: Tom Christian (415) 677-0424, Tom Niu (415) 568-3423;Tim Garlick (415) 568-34166 2.76± ac. 60 Christopher Ct. In Contract SCHOOL DISTRICT SITE available.Property consists of a functionallyobsolete, unoccupied school facilitysituated on a predominately downsloping,irregular-shaped parcelsuitable for single-family detacheddevelopment.Contact:Bruce Paris, CB Richard Ellis, (650) 577-29336 1.4 ac. Serramonte Blvd.(south side)$3,970,000NegotiablePRE-APPROVED FOR HOTEL OROFFICE. Owner will consider all offers.Contact:Bruce Paris, CB Richard Ellis, (650) 577-29336 4.8± ac. Serramonte Blvd.(at Callan Blvd.)Contact: Bruce Paris, CB Richard Ellis, (650) 577-2933$16,000,000NegotiableAPPPROVED FOR 200-UNIT MULTI-FAMILY development (subject totentative map). Single up-slopingparcel. Proximity to Serramonte Ctr.w/direct Hwy. 1 & 280 access.
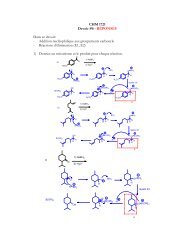
14.a. Draw the two chair conformations of trans-1-tert-butyl-2-ethylcyclohexane. (5 points)b. For each structure, label the substituents as being axial or equatorial. (2points) – 1 per errorc. Identify the most stable and least stable conformation. (1 point)/5:1 point: chairs well-drawn1 point: right molecule1 point: both conformations drawn (many people drew the enantiomer)2 points: orientation of the substituentsaxialaxialequatorialequatorialleast stablemost stable115. Consider the Newman projection of propan-1-ol down the C1-C2 bond.OHa. Draw and name the Newman projection of the least stable conformation (3points)HO CH 3HH HHeclipsed1 required conformation1 correct molecule1 name of conformationb. Draw and name the Newman projection of the most stable conformation(3 points)HHCH 3HHOHanti1 required conformation1 correct molecule1 name of conformation5
16. Ephedrine has the structure shown below but with the (1R, 2S) configuration.a. Draw its structure with the correct configurations at the stereocentres. Thepriorities given to each group to determine the correct structure must beindicated (redrawing the structure for each chiral centre helps). (4 points)b. Draw the enantiomer of ephedrine. (2 points)a. 1 point per chiral centre drawn with correct stereochemistry2 points for the priorities being correctly assignedOHHN14OHHHOH3R 2HNCH 3CH2 S 334 H HN1 CH 3b.OHCH 3HN2 points (- 1 per error)BONUS!Is the following molecule chiral? Explain or show clearly how you came to thatconclusion (2 points)HH 3 CClC C CHClCH1CCHCH 3YES, because the molecule is not superimposable on its mirrorimage.1ORYES, because the molecule does not contain a plane of symmetry (with justification)6