CHM 1321 A Assignment 1 - Université d'Ottawa
CHM 1321 A Assignment 1 - Université d'Ottawa
CHM 1321 A Assignment 1 - Université d'Ottawa
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
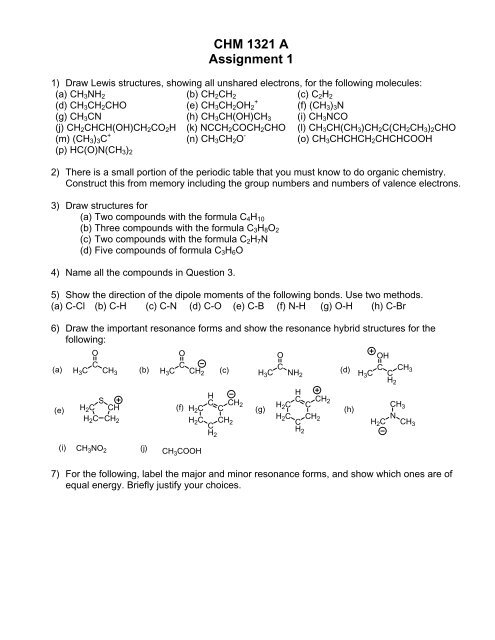
<strong>CHM</strong> <strong>1321</strong> A<strong>Assignment</strong> 11) Draw Lewis structures, showing all unshared electrons, for the following molecules:(a) CH 3 NH 2 (b) CH 2 CH 2 (c) C 2 H 2(d) CH 3 CH 2 CHO+(e) CH 3 CH 2 OH 2 (f) (CH 3 ) 3 N(g) CH 3 CN (h) CH 3 CH(OH)CH 3 (i) CH 3 NCO(j) CH 2 CHCH(OH)CH 2 CO 2 H (k) NCCH 2 COCH 2 CHO (l) CH 3 CH(CH 3 )CH 2 C(CH 2 CH 3 ) 2 CHO(m) (CH 3 ) 3 C + (n) CH 3 CH 2 O - (o) CH 3 CHCHCH 2 CHCHCOOH(p) HC(O)N(CH 3 ) 22) There is a small portion of the periodic table that you must know to do organic chemistry.Construct this from memory including the group numbers and numbers of valence electrons.3) Draw structures for(a) Two compounds with the formula C 4 H 10(b) Three compounds with the formula C 3 H 8 O 2(c) Two compounds with the formula C 2 H 7 N(d) Five compounds of formula C 3 H 6 O4) Name all the compounds in Question 3.5) Show the direction of the dipole moments of the following bonds. Use two methods.(a) C-Cl (b) C-H (c) C-N (d) C-O (e) C-B (f) N-H (g) O-H (h) C-Br6) Draw the important resonance forms and show the resonance hybrid structures for thefollowing:OO(a) H 3 C C CH 3 (b) H 3 C C CH 2 (c) H (d)3 C C NH 2OOHH 3 C C CH2CH 3(e)SH 2 C CHH 2 C CH 2H(f) H 2 CC CCH 2 (g)H 2 C CH 2 CH2HC CHH 2 C C 2H 2 C CH 2 CH2(h)CH 3NH 2 C CH 3(i) CH 3 NO 2 (j) CH 3 COOH7) For the following, label the major and minor resonance forms, and show which ones are ofequal energy. Briefly justify your choices.
(a)H 3 C H C C NH 3 C H C C N(b)OH 3 C C CHHC CH3OH 3 C C CHHC CH3OH 3 C C CHHC CH3(c)OOH 3 C C CHCH2CH 3OOH 3 C C CHC CH2CH 3OOH 3 C C CHC CH2CH 3(d)H 3 CH 2C CNH 2NH 2H 3 CH 2C CNH 2NH 2H 3 CH 2C CNH 28) For each pair of ions, determine which is more stable. Justify your answer in each case.(a)H 3 C H C CH 3 or H 3 C H C OCH 3NH 2(b) H 2 C H C H C CH 3or H 2 C H CH 2C CH 2(c) CH 2 CH 3or CH 2 CN(d)H 2 CH 2 CH 2C CH 2CorCH 2CHH 2 CH 2 CH 2C CH 2CHCHCH(e)H 3 C CH 3NorCH 3 C CH 3H 3 C CH 3CHCH 3 C CH 3(f) CH 3 CO 2or CH 3 CH 2 O(e)HCHCHC CH 2CorCHCHH 2 CH 2C CH 2CH 2 CCH2CH
9) Draw the shape of s and p orbitals including phasing. Show the resulting shapes following sp,sp 2 and sp 3 hybridization.10) Draw complete molecular orbital structures for the following molecules using the LCAOmethod. Label the atomic orbitals used to make bonds. Label the bonds. Indicate thegeometry of each atom.(a) CH 3 CH 2 NH 2 (b) CH 3 CO 2 H (c) CH 3 CHCHCH 2 CH 3 (d) CH 3 NO 2 (e) CH 3 CN(f) CH 3 OCH 3