Ranking Nucleophiles and Leaving Groups, Hammond's Postulate
Ranking Nucleophiles and Leaving Groups, Hammond's Postulate
Ranking Nucleophiles and Leaving Groups, Hammond's Postulate
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
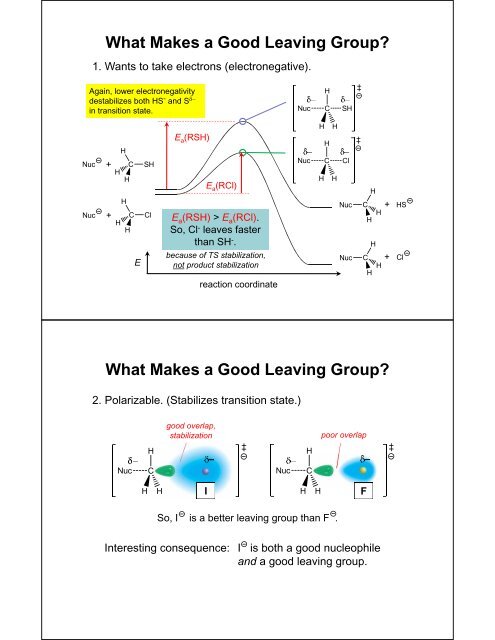
What Makes a Good <strong>Leaving</strong> Group?1. Wants to take electrons (electronegative).Again, lower electronegativitydestabilizes both HS - <strong>and</strong> S in transition state.E a (RSH)+E a (RCl)+EE a (RSH) > E a (RCl).So, Cl - leaves fasterthan SH - .because of TS stabilization,not product stabilizationreaction coordinate++What Makes a Good <strong>Leaving</strong> Group?2. Polarizable. (Stabilizes transition state.)good overlap,stabilizationpoor overlapIFSo, I is a better leaving group than F .Interesting consequence: I is both a good nucleophile<strong>and</strong> a good leaving group.