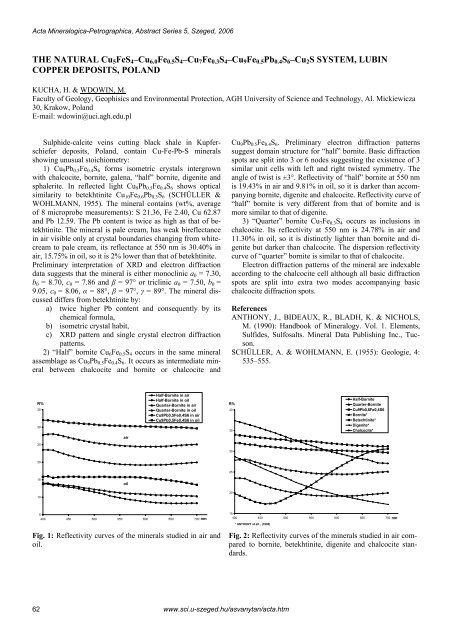
Acta Mineralogica-Petrographica, Abstract Series 5, Szeged, 2006<strong>THE</strong> NATURAL Cu 5 FeS 4 –Cu 6.0 Fe 0.5 S 4 –Cu 7 Fe 0.3 S 4 –Cu 9 Fe 0.5 Pb 0.4 S 6 –Cu 2 S SYSTEM, LUB<strong>IN</strong>COPPER DEPOSITS, POLANDKUCHA, H. & WDOW<strong>IN</strong>, M.Faculty of Geology, Geophisics and Environmental Protection, AGH University of Science and Technology, Al. Mickiewicza30, Krakow, PolandE-mail: wdowin@uci.agh.edu.plSulphide-calcite veins cutting black shale in Kupferschieferdeposits, Poland, contain Cu-Fe-Pb-S mineralsshowing unusual stoichiometry:1) Cu 9 Pb 0.5 Fe 0.4 S 6 forms isometric crystals intergrownwith chalcocite, bornite, galena, “half” bornite, digenite andsphalerite. In reflected light Cu 9 Pb 0.5 Fe 0.4 S 6 shows opticalsimilarity to betekhtinite Cu 10 Fe 0.6 Pb 0.3 S 6 (SCHÜLLER &WOHLMANN, 1955). The mineral contains (wt%, averageof 8 microprobe measurements): S 21.36, Fe 2.40, Cu 62.87and Pb 12.59. The Pb content is twice as high as that of betekhtinite.The mineral is pale cream, has weak bireflectancein air visible only at crystal boundaries changing from whitecreamto pale cream, its reflectance at 550 nm is 30.40% inair, 15.75% in oil, so it is 2% lower than that of betekhtinite.Preliminary interpretation of XRD and electron diffractiondata suggests that the mineral is either monoclinic a 0 = 7.30,b 0 = 8.70, c 0 = 7.86 and β = 97° or triclinic a 0 = 7.50, b 0 =9.05, c 0 = 8.06, α = 88°, β = 97°, γ = 89°. The mineral discusseddiffers from betekhtinite by:a) twice higher Pb content and consequently by itschemical formula,b) isometric crystal habit,c) XRD pattern and single crystal electron diffractionpatterns.2) “Half” bornite Cu 6 Fe 0.5 S 4 occurs in the same mineralassemblage as Cu 9 Pb 0.5 Fe 0.4 S 6 . It occurs as intermediate mineralbetween chalcocite and bornite or chalcocite andCu 9 Pb 0.5 Fe 0.4 S 6 . Preliminary electron diffraction patternssuggest domain structure for “half” bornite. Basic diffractionspots are split into 3 or 6 nodes suggesting the existence of 3similar unit cells with left and right twisted symmetry. Theangle of twist is ±3°. Reflectivity of “half” bornite at 550 nmis 19.43% in air and 9.81% in oil, so it is darker than accompanyingbornite, digenite and chalcocite. Reflectivity curve of“half” bornite is very different from that of bornite and ismore similar to that of digenite.3) “Quarter” bornite Cu 7 Fe 0.3 S 4 occurs as inclusions inchalcocite. Its reflectivity at 550 nm is 24.78% in air and11.30% in oil, so it is distinctly lighter than bornite and digenitebut darker than chalcocite. The dispersion reflectivitycurve of “quarter” bornite is similar to that of chalcocite.Electron diffraction patterns of the mineral are indexableaccording to the chalcocite cell although all basic diffractionspots are split into extra two modes accompanying basicchalcocite diffraction spots.ReferencesANTHONY, J., BIDEAUX, R., BLADH, K. & NICHOLS,M. (1990): Handbook of Mineralogy. Vol. 1. Elements,Sulfides, Sulfosalts. Mineral Data Publishing Inc., Tucson.SCHÜLLER, A. & WOHLMANN, E. (1955): Geologie, 4:535–555.R%3530Half-Bornite in airHalf-Bornite in oilQuarter-Bornite in airQuarter-Bornite in oilCu9Pb0,5Fe0,4S6 in airCu9Pb0,5Fe0,4S6 in oilR%4035Half-BorniteQuarter-BorniteCu9Pb0,6Fe0,4S6Bornite*Betechtinite*Digenite*Chalcocite*air2530202515oil10205400 450 500 550 600 650 700 nmFig. 1: Reflectivity curves of the minerals studied in air andoil.15400 450 500 550 600 650 700 nm* ANTHONY at all ., (1990)Fig. 2: Reflectivity curves of the minerals studied in air comparedto bornite, betekhtinite, digenite and chalcocite standards.62www.sci.u-szeged.hu/asvanytan/acta.htm
Acta Mineralogica-Petrographica, Abstract Series 5, Szeged, 2006NATIVE COPPER OF <strong>THE</strong> UKRA<strong>IN</strong>IAN <strong>CARPATHIANS</strong> AND ADJACENT AREASKVASNYTSYA, I.Geological Faculty of Taras Shevchenko Kyiv National University, Vasyl’kivs’ka Street 90, 03022 Kyiv, UkraineE-mail: irenek@bigmir.netNative copper is a rare mineral of the UkrainianCarpathians and a widespread mineral of their adjacent areas,especially in the Volyn’ region (LAZARENKO et al., 1960;LAZARENKO et al., 1962; LAZARENKO et al., 1963;KHRUSHCHOV et al., 1977; SHCHERBAK (ed.), 1990). Inthe Ukrainian Carpathians copper is known as mineral ofoxidation zones in some ore deposits and occurrences as wellas in Cu-bearing sandstones and also in the alluvium ofCarpathian rivers. In the Volyn’ region copper occurs inmany localities of Cu-bearing basalts, tuffs, volcanic breccia,and sandstones. Short description of native copper from theseoccurrences is given in Table 1.ReferencesLAZARENKO, E.K., GAB<strong>IN</strong>ET, M.P. & SLIVKO, E.P.(1962): Mineralogy of sedimentary formations of thePrecarpathians. L’viv: L’viv State University, 482 p. (inUkrainian).LAZARENKO, E.K., LAZARENKO, E.A.,BARYSHNIKOV, E.K. & MALYG<strong>IN</strong>A, O.A. (1963):Mineralogy of the Trans-Carpathians. L’vov: L’vоv StateUniversity: 614 p. (in Russian).LAZARENKO, E.K., MATKOVSKY, O.I., V<strong>IN</strong>AR, O.N.,SHASHK<strong>IN</strong>A, V.P. & GNATIV G.M. (1960):Mineralogy of igneous complexes of the WesternVolynia. L’viv: L’viv State University, 509 p. (inUkrainian).KHRUSHCHOV, D.P., NECHAEV, YU.A., KARDASH,V.T. AND GALIY, S.A. (1977): Copper ores of stratabound type in deposits paragenetically connected withevaporites of the Ukraine, Kiev: IGPHM, 47 p. (inRussian).SHCHERBAK, N.P. (ed.) (1990): Minerals of the UkrainianCarpathians. Native elements, tellurides and sulphides,Kiev: Naukova dumka, 150 p. (in Russian).Table 1: Characteristics of native copper of the Ukrainian Carpathians and adjacent areas.Region, area,districtRocks, ores, theiragesMineral associationsDistribution, formand size of copperCompositionof copperTrans-Carpathians:Beregove polymetallicdepositZone of oxidation ofsulphide ores inNeogene rhyolite tuffsSphalerite, galena,cerussiteRare mineral. Verytine and thin films onsphalerite crystals99.1–99.3%Cu, traces ofAg and SbUkrainianCarpathians:Marmaroshcrystalline massif,the Rakhiv districtUkrainianCarpathians:Marmaroshcrystalline massif,the basin of BilyCheremosh andChorny CheremoshriversPrecarpathians: thebasin of BystrytsyaNadvirna and Prutrivers (Nadvirnaand Delyatyndistrict)Northern-westernpart of the Volyn’–Podillya plate:Volyn’ regionPyritiferouspolymetallicores inRiphean-Paleozoicmetamorphic rocks ofBily Potik andDilovets’ suitesModern alluvialsedimentsNeogene cupriferoussandstones and claysof Nyzhny StebnyksuiteVendian volcanic andvolcano-sedimentaryrocks (basalts, theirlava and tuff breccias,tuffs, quartz veins,sandstones)Sphalerite, galena,pyrite, chalcopyrite,pyrrhotine, quartz,calciteCuprite, chalcocite,hematite, sphalerite,galena, cinnabar,malachitePyrite, chalcocite,cuprite, covellite,tenorite, chalcopyrite,malachite, azurite,hydroxides of ironQuartz, chalcedony,calcite, chlorite,analcime, zeolites,chalcocite, cuprite,chalcopyrite, nativeAg, native Fe,malachite, azuriteRare mineral. Smallisometric grains,sometimes up to 2–3mm.Rare mineral. Smalltable like grains,sometimes up to 5–7mmRare mineral. Verysmall films and tablelike grains, 0.01–0.1mm in sizeWidespread mineral.Xenomorphic grains,hypidiomorphic andidiomorphic crystals,from 0.1 to 140 mmin size99.0% Cu,traces of Fe,Ag, Sb and V99.0% Cu,traces of Feand Ag98.9–99.3%Cu, traces ofFe and Ag99.5% Cu, upto 1.04% Fe,up to 0.37%Ag, traces ofAuwww.sci.u-szeged.hu/asvanytan/acta.htm 63
- Page 1:
MSCC33 rd MINERAL SCIENCES IN THE C
- Page 5 and 6:
Acta Mineralogica-Petrographica, Ab
- Page 7 and 8:
Acta Mineralogica-Petrographica, Ab
- Page 9 and 10:
Acta Mineralogica-Petrographica, Ab
- Page 11 and 12: Acta Mineralogica-Petrographica, Ab
- Page 13 and 14: Acta Mineralogica-Petrographica, Ab
- Page 15 and 16: Acta Mineralogica-Petrographica, Ab
- Page 17 and 18: Acta Mineralogica-Petrographica, Ab
- Page 19 and 20: Acta Mineralogica-Petrographica, Ab
- Page 21 and 22: Acta Mineralogica-Petrographica, Ab
- Page 23 and 24: Acta Mineralogica-Petrographica, Ab
- Page 25 and 26: Acta Mineralogica-Petrographica, Ab
- Page 27 and 28: Acta Mineralogica-Petrographica, Ab
- Page 29 and 30: Acta Mineralogica-Petrographica, Ab
- Page 31 and 32: Acta Mineralogica-Petrographica, Ab
- Page 33 and 34: Acta Mineralogica-Petrographica, Ab
- Page 35 and 36: Acta Mineralogica-Petrographica, Ab
- Page 37 and 38: Acta Mineralogica-Petrographica, Ab
- Page 39 and 40: Acta Mineralogica-Petrographica, Ab
- Page 41 and 42: Acta Mineralogica-Petrographica, Ab
- Page 43 and 44: Acta Mineralogica-Petrographica, Ab
- Page 45 and 46: Acta Mineralogica-Petrographica, Ab
- Page 47 and 48: Acta Mineralogica-Petrographica, Ab
- Page 49 and 50: Acta Mineralogica-Petrographica, Ab
- Page 51 and 52: Acta Mineralogica-Petrographica, Ab
- Page 53 and 54: Acta Mineralogica-Petrographica, Ab
- Page 55 and 56: Acta Mineralogica-Petrographica, Ab
- Page 57 and 58: Acta Mineralogica-Petrographica, Ab
- Page 59 and 60: Acta Mineralogica-Petrographica, Ab
- Page 61: Acta Mineralogica-Petrographica, Ab
- Page 65 and 66: Acta Mineralogica-Petrographica, Ab
- Page 67 and 68: Acta Mineralogica-Petrographica, Ab
- Page 69 and 70: Acta Mineralogica-Petrographica, Ab
- Page 71 and 72: Acta Mineralogica-Petrographica, Ab
- Page 73 and 74: Acta Mineralogica-Petrographica, Ab
- Page 75 and 76: Acta Mineralogica-Petrographica, Ab
- Page 77 and 78: Acta Mineralogica-Petrographica, Ab
- Page 79 and 80: Acta Mineralogica-Petrographica, Ab
- Page 81 and 82: Acta Mineralogica-Petrographica, Ab
- Page 83 and 84: Acta Mineralogica-Petrographica, Ab
- Page 85 and 86: Acta Mineralogica-Petrographica, Ab
- Page 87 and 88: Acta Mineralogica-Petrographica, Ab
- Page 89 and 90: Acta Mineralogica-Petrographica, Ab
- Page 91 and 92: Acta Mineralogica-Petrographica, Ab
- Page 93 and 94: Acta Mineralogica-Petrographica, Ab
- Page 95 and 96: Acta Mineralogica-Petrographica, Ab
- Page 97 and 98: Acta Mineralogica-Petrographica, Ab
- Page 99 and 100: Acta Mineralogica-Petrographica, Ab
- Page 101 and 102: Acta Mineralogica-Petrographica, Ab
- Page 103 and 104: Acta Mineralogica-Petrographica, Ab
- Page 105 and 106: Acta Mineralogica-Petrographica, Ab
- Page 107 and 108: Acta Mineralogica-Petrographica, Ab
- Page 109 and 110: Acta Mineralogica-Petrographica, Ab
- Page 111 and 112: Acta Mineralogica-Petrographica, Ab
- Page 113 and 114:
Acta Mineralogica-Petrographica, Ab
- Page 115 and 116:
Acta Mineralogica-Petrographica, Ab
- Page 117 and 118:
Acta Mineralogica-Petrographica, Ab
- Page 119 and 120:
Acta Mineralogica-Petrographica, Ab
- Page 121 and 122:
Acta Mineralogica-Petrographica, Ab
- Page 123 and 124:
Acta Mineralogica-Petrographica, Ab
- Page 125 and 126:
Acta Mineralogica-Petrographica, Ab
- Page 127 and 128:
Acta Mineralogica-Petrographica, Ab
- Page 129 and 130:
Acta Mineralogica-Petrographica, Ab
- Page 131 and 132:
Acta Mineralogica-Petrographica, Ab
- Page 133 and 134:
Acta Mineralogica-Petrographica, Ab