Effect of pH on the formation of complex compounds with Schiff ...
Effect of pH on the formation of complex compounds with Schiff ...
Effect of pH on the formation of complex compounds with Schiff ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
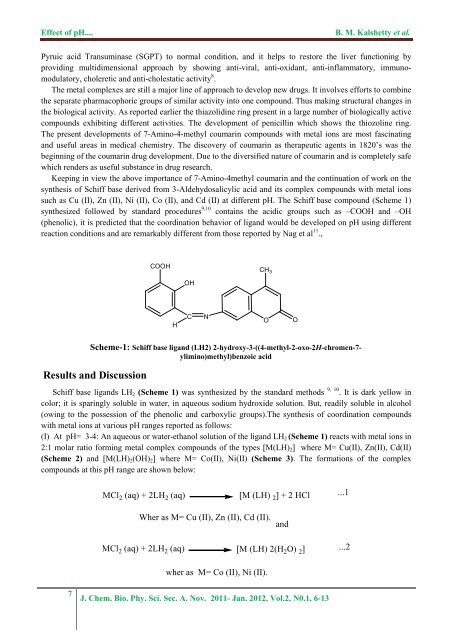
<str<strong>on</strong>g>Effect</str<strong>on</strong>g> <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>pH</str<strong>on</strong>g>....B. M. Kalshetty et al.Pyruic acid Transuminase (SGPT) to normal c<strong>on</strong>diti<strong>on</strong>, and it helps to restore <strong>the</strong> liver functi<strong>on</strong>ing byproviding multidimensi<strong>on</strong>al approach by showing anti-viral, anti-oxidant, anti-inflammatory, immunomodulatory,choleretic and anti-cholestatic activity 8 .The metal <strong>complex</strong>es are still a major line <str<strong>on</strong>g>of</str<strong>on</strong>g> approach to develop new drugs. It involves efforts to combine<strong>the</strong> separate pharmacophoric groups <str<strong>on</strong>g>of</str<strong>on</strong>g> similar activity into <strong>on</strong>e compound. Thus making structural changes in<strong>the</strong> biological activity. As reported earlier <strong>the</strong> thiazolidine ring present in a large number <str<strong>on</strong>g>of</str<strong>on</strong>g> biologically active<strong>compounds</strong> exhibiting different activities. The development <str<strong>on</strong>g>of</str<strong>on</strong>g> penicillin which shows <strong>the</strong> thiozoline ring.The present developments <str<strong>on</strong>g>of</str<strong>on</strong>g> 7-Amino-4-methyl coumarin <strong>compounds</strong> <strong>with</strong> metal i<strong>on</strong>s are most fascinatingand useful areas in medical chemistry. The discovery <str<strong>on</strong>g>of</str<strong>on</strong>g> coumarin as <strong>the</strong>rapeutic agents in 1820’s was <strong>the</strong>beginning <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> coumarin drug development. Due to <strong>the</strong> diversified nature <str<strong>on</strong>g>of</str<strong>on</strong>g> coumarin and is completely safewhich renders as useful substance in drug research.Keeping in view <strong>the</strong> above importance <str<strong>on</strong>g>of</str<strong>on</strong>g> 7-Amino-4methyl coumarin and <strong>the</strong> c<strong>on</strong>tinuati<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> work <strong>on</strong> <strong>the</strong>syn<strong>the</strong>sis <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>Schiff</strong> base derived from 3-Aldehydosalicylic acid and its <strong>complex</strong> <strong>compounds</strong> <strong>with</strong> metal i<strong>on</strong>ssuch as Cu (II), Zn (II), Ni (II), Co (II), and Cd (II) at different <str<strong>on</strong>g>pH</str<strong>on</strong>g>. The <strong>Schiff</strong> base compound (Scheme 1)syn<strong>the</strong>sized followed by standard procedures 9,10 c<strong>on</strong>tains <strong>the</strong> acidic groups such as –COOH and –OH(phenolic), it is predicted that <strong>the</strong> coordinati<strong>on</strong> behavior <str<strong>on</strong>g>of</str<strong>on</strong>g> ligand would be developed <strong>on</strong> <str<strong>on</strong>g>pH</str<strong>on</strong>g> using differentreacti<strong>on</strong> c<strong>on</strong>diti<strong>on</strong>s and are remarkably different from those reported by Nag et al 11 .,COOHCH 3OHHCNOOScheme-1: <strong>Schiff</strong> base ligand (LH2) 2-hydroxy-3-((4-methyl-2-oxo-2H-chromen-7-ylimino)methyl)benzoic acidResults and Discussi<strong>on</strong><strong>Schiff</strong> base ligands LH 2 (Scheme 1) was syn<strong>the</strong>sized by <strong>the</strong> standard methods 9, 10 . It is dark yellow incolor; it is sparingly soluble in water, in aqueous sodium hydroxide soluti<strong>on</strong>. But, readily soluble in alcohol(owing to <strong>the</strong> possessi<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> phenolic and carboxylic groups).The syn<strong>the</strong>sis <str<strong>on</strong>g>of</str<strong>on</strong>g> coordinati<strong>on</strong> <strong>compounds</strong><strong>with</strong> metal i<strong>on</strong>s at various <str<strong>on</strong>g>pH</str<strong>on</strong>g> ranges reported as follows:(I) At <str<strong>on</strong>g>pH</str<strong>on</strong>g>= 3-4: An aqueous or water-ethanol soluti<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> ligand LH 2 (Scheme 1) reacts <strong>with</strong> metal i<strong>on</strong>s in2:1 molar ratio forming metal <strong>complex</strong> <strong>compounds</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> types [M(LH) 2 ] where M= Cu(II), Zn(II), Cd(II)(Scheme 2) and [M(LH) 2 (OH) 2 ] where M= Co(II), Ni(II) (Scheme 3). The formati<strong>on</strong>s <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> <strong>complex</strong><strong>compounds</strong> at this <str<strong>on</strong>g>pH</str<strong>on</strong>g> range are shown below:MCl 2 (aq) + 2LH 2 (aq)[M (LH) 2 ] + 2 HCl...1Wher as M= Cu (II), Zn (II), Cd (II).andMCl 2 (aq) + 2LH 2 (aq) [M (LH) 2(H 2 O) 2 ]...2wher as M= Co (II), Ni (II).7J. Chem. Bio. Phy. Sci. Sec. A. Nov. 2011- Jan. 2012, Vol.2, N0.1, 6-13