Effect of pH on the formation of complex compounds with Schiff ...
Effect of pH on the formation of complex compounds with Schiff ...
Effect of pH on the formation of complex compounds with Schiff ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
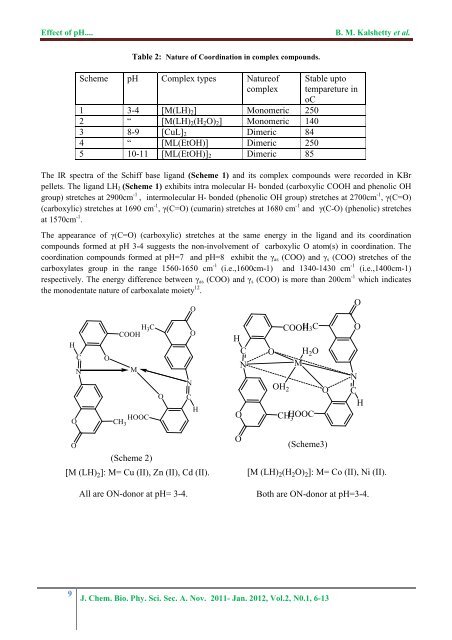
<str<strong>on</strong>g>Effect</str<strong>on</strong>g> <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>pH</str<strong>on</strong>g>....B. M. Kalshetty et al.Table 2: Nature <str<strong>on</strong>g>of</str<strong>on</strong>g> Coordinati<strong>on</strong> in <strong>complex</strong> <strong>compounds</strong>.Scheme <str<strong>on</strong>g>pH</str<strong>on</strong>g> Complex types Nature<str<strong>on</strong>g>of</str<strong>on</strong>g><strong>complex</strong>1 3-4 [M(LH) 2 ] M<strong>on</strong>omeric 2502 “ [M(LH) 2 (H 2 O) 2 ] M<strong>on</strong>omeric 1403 8-9 [CuL] 2 Dimeric 844 “ [ML(EtOH)] Dimeric 2505 10-11 [ML(EtOH)] 2 Dimeric 85Stable uptotempareture inoCThe IR spectra <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> <strong>Schiff</strong> base ligand (Scheme 1) and its <strong>complex</strong> <strong>compounds</strong> were recorded in KBrpellets. The ligand LH 2 (Scheme 1) exhibits intra molecular H- b<strong>on</strong>ded (carboxylic COOH and phenolic OHgroup) stretches at 2900cm -1 , intermolecular H- b<strong>on</strong>ded (phenolic OH group) stretches at 2700cm -1 , γ(C=O)(carboxylic) stretches at 1690 cm -1 , γ(C=O) (cumarin) stretches at 1680 cm -1 and γ(C-O) (phenolic) stretchesat 1570cm -1 .The appearance <str<strong>on</strong>g>of</str<strong>on</strong>g> γ(C=O) (carboxylic) stretches at <strong>the</strong> same energy in <strong>the</strong> ligand and its coordinati<strong>on</strong><strong>compounds</strong> formed at <str<strong>on</strong>g>pH</str<strong>on</strong>g> 3-4 suggests <strong>the</strong> n<strong>on</strong>-involvement <str<strong>on</strong>g>of</str<strong>on</strong>g> carboxylic O atom(s) in coordinati<strong>on</strong>. Thecoordinati<strong>on</strong> <strong>compounds</strong> formed at <str<strong>on</strong>g>pH</str<strong>on</strong>g>=7 and <str<strong>on</strong>g>pH</str<strong>on</strong>g>=8 exhibit <strong>the</strong> γ as (COO) and γ s (COO) stretches <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong>carboxylates group in <strong>the</strong> range 1560-1650 cm -1 (i.e.,1600cm-1) and 1340-1430 cm -1 (i.e.,1400cm-1)respectively. The energy difference between γ as (COO) and γ s (COO) is more than 200cm -1 which indicates<strong>the</strong> m<strong>on</strong>odentate nature <str<strong>on</strong>g>of</str<strong>on</strong>g> carboxalate moiety 12 .OOHCNOOH 3 CCOOHMHOOCCH 3OONCHHCONCOOHH 3 CO H 2 OMOH 2CHHOOC3ONOCHO(Scheme 2)[M (LH) 2 ]: M= Cu (II), Zn (II), Cd (II).All are ON-d<strong>on</strong>or at <str<strong>on</strong>g>pH</str<strong>on</strong>g>= 3-4.O(Scheme3)[M (LH) 2 (H 2 O) 2 ]: M= Co (II), Ni (II).Both are ON-d<strong>on</strong>or at <str<strong>on</strong>g>pH</str<strong>on</strong>g>=3-4.9J. Chem. Bio. Phy. Sci. Sec. A. Nov. 2011- Jan. 2012, Vol.2, N0.1, 6-13