Effect of pH on the formation of complex compounds with Schiff ...
Effect of pH on the formation of complex compounds with Schiff ...
Effect of pH on the formation of complex compounds with Schiff ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
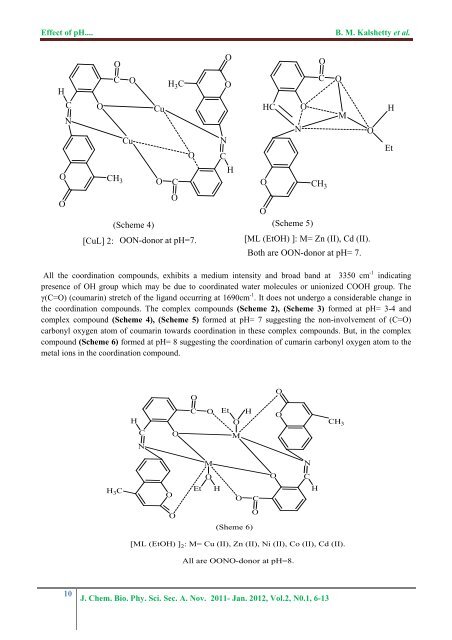
<str<strong>on</strong>g>Effect</str<strong>on</strong>g> <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>pH</str<strong>on</strong>g>....B. M. Kalshetty et al.HCNOOOC OOCuCuH 3 COCH 3 O CO(Scheme 4)[CuL] 2: OON-d<strong>on</strong>or at <str<strong>on</strong>g>pH</str<strong>on</strong>g>=7.OONCHOC OHC OMNOO CH 3O(Scheme 5)[ML (EtOH) ]: M= Zn (II), Cd (II).Both are OON-d<strong>on</strong>or at <str<strong>on</strong>g>pH</str<strong>on</strong>g>= 7.HEtAll <strong>the</strong> coordinati<strong>on</strong> <strong>compounds</strong>, exhibits a medium intensity and broad band at 3350 cm -1 indicatingpresence <str<strong>on</strong>g>of</str<strong>on</strong>g> OH group which may be due to coordinated water molecules or uni<strong>on</strong>ized COOH group. Theγ(C=O) (coumarin) stretch <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> ligand occurring at 1690cm -1 . It does not undergo a c<strong>on</strong>siderable change in<strong>the</strong> coordinati<strong>on</strong> <strong>compounds</strong>. The <strong>complex</strong> <strong>compounds</strong> (Scheme 2), (Scheme 3) formed at <str<strong>on</strong>g>pH</str<strong>on</strong>g>= 3-4 and<strong>complex</strong> compound (Scheme 4), (Scheme 5) formed at <str<strong>on</strong>g>pH</str<strong>on</strong>g>= 7 suggesting <strong>the</strong> n<strong>on</strong>-involvement <str<strong>on</strong>g>of</str<strong>on</strong>g> (C=O)carb<strong>on</strong>yl oxygen atom <str<strong>on</strong>g>of</str<strong>on</strong>g> coumarin towards coordinati<strong>on</strong> in <strong>the</strong>se <strong>complex</strong> <strong>compounds</strong>. But, in <strong>the</strong> <strong>complex</strong>compound (Scheme 6) formed at <str<strong>on</strong>g>pH</str<strong>on</strong>g>= 8 suggesting <strong>the</strong> coordinati<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> cumarin carb<strong>on</strong>yl oxygen atom to <strong>the</strong>metal i<strong>on</strong>s in <strong>the</strong> coordinati<strong>on</strong> compound.OOHCNOCOEtOMHOCH 3MNOOCH 3 COEtHOCHOO(Sheme 6)[ML (EtOH) ] 2 : M= Cu (II), Zn (II), Ni (II), Co (II), Cd (II).All are OONO-d<strong>on</strong>or at <str<strong>on</strong>g>pH</str<strong>on</strong>g>=8.10J. Chem. Bio. Phy. Sci. Sec. A. Nov. 2011- Jan. 2012, Vol.2, N0.1, 6-13