Chemistry 1011
Chemistry 1011
Chemistry 1011
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
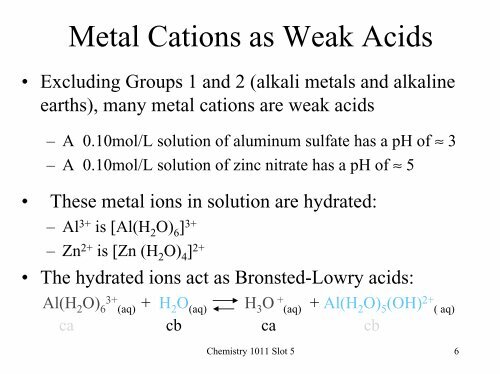
Metal Cations as Weak Acids• Excluding Groups 1 and 2 (alkali metals and alkalineearths), many metal cations are weak acids– A 0.10mol/L solution of aluminum sulfate has a pH of ≈ 3– A 0.10mol/L solution of zinc nitrate has a pH of ≈ 5• These metal ions in solution are hydrated:–Al 3+ is [Al(H 2 O) 6 ] 3+–Zn 2+ is [Zn (H 2 O) 4 ] 2+• The hydrated ions act as Bronsted-Lowry acids:Al(H 2 O) 63+(aq) + H 2 O (aq) H 3 O + (aq) + Al(H 2 O) 5 (OH) 2+ ( aq)ca cb ca cb<strong>Chemistry</strong> <strong>1011</strong> Slot 5 6